Document Type : Original Research Article
Authors
1 Department of Obstetrics and Gynaecology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
2 Department of Radiotherapy, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
Abstract
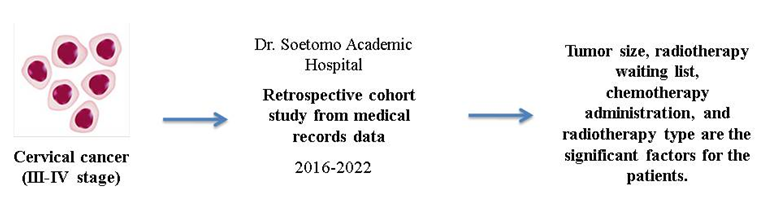
The aims of this article was to determine the correlation among clinical characteristics and therapeutic treatments on disease free survival (DFS) and 5 years survival in stage III-IV cervical cancer patients with limited radiotherapy facility. This was a retrospective cohort study of all patients admitted to our hospital from 2016-2022. Data on clinical characteristics were collected from medical records. Statistical analysis was performed to determine the relationship between therapeutic treatments and clinical characteristics, including age, histopathology, tumor size, and patient outcomes. Total sample was 216 patients. Patient with tumor size <6 cm had better survival rate and DFS than tumor size >6 cm (24 months vs. 21 months and 22 months vs. 12 months), radiotherapy waiting list <4 months showed better survival rate and DFS than patients who waited >4 months (33 months vs. 24 months and 24 months vs. 16 months), patients who received chemotherapy while waiting for radiotherapy had better survival rate and DFS than patients who did not (24 months vs. 10 months and 22 months vs. 10 months), and patients who continued ER AFL had better survival rate and DFS than patients who just received ER (44 months vs. 24 months and 34 months vs. 16 months). Tumor size, radiotherapy waiting list, chemotherapy administration, and type of radiotherapy are the most significant factors for outcome patients of stage III-IV cervical cancer.
Graphical Abstract
Keywords
Introduction
Cervical cancer is a major public health problem worldwide [1-3]. Cervical cancer is the third most common gynecological malignancy in women worldwide [4-5]. Approximately 500,000 new cases and 237,500 deaths from cervical cancer occurred each year. Global Cancer Observatory (GLOBOCAN) in 2020, cervical cancer has recorded around 604,000 new cases and 342,000 deaths worldwide and the fourth case of cancer in women in the world. Countries without good cervical cancer screening and prevention programs still confirm that cervical cancer as the highest cause of female mortality by gynecological cancers [6-8]. Data on gynecological oncology clinic visits at Dr. Soetomo Hospital (the second largest city in Indonesia, Surabaya) that have been documented since 2016, show the number of new patients with cervical cancer in 2016 as many as 858 cases (68.7% from all gynecologic cancer), in 2017 as many as 692 cases (49.6%) with total number of new patients with stage III-IV cervical cancer in 2016-2017 reaching 1075 cases. Platinum-based Concurrent chemoradiation (CCRT) is gold standard for locally advanced stage cervical cancer [9]. In Indonesia, not all hospitals can apply CCRT because the number of cervical cancer patients is very large, plus the limitations of radiotherapy facility, so modifications are needed in the management of locally advanced stage cervical cancer. Dr. Soetomo hospital in 2016-2017 had 4 external radiation devices and 1 brachytherapy device to serve all cancer patients.The waiting list of radiotherapy approximately 4-5 months. While waiting for the radiotherapy, patients received chemotherapy.This research to determine the relationship between therapeutic treatments including radiotherapy waiting list, administration of chemotherapy while waiting radiation, and type of radiotherapy, and clinical characteristics including age, histopathology, tumor size, and patient outcomes.
Experimental
Study Design
This was an analytical observational study that used secondary data or medical records of patients diagnosed with stage III and IV of cervical cancer in January 2016 to December 2017 who underwent radiotherapy at our hospital. This study was conducted under the approval and supervision of the Ethics Committee of Dr. Soetomo Academic Hospital (registration number 1998/104/4/II/2023).
Data Retrieval
Clinical characteristics were assessed, including age, histopathology, tumor size, and therapeutic treatment (waiting list of radiotherapy, administration of chemotherapy while waiting radiation, and type of radiotherapy). At our institution, we cannot do CCRT so that the treatment of stage III-IV cervical cancer patients was given chemotherapy (cisplatin 50 mg/m2 and 3 weekly) while waiting for the radiotherapy schedule for approximately 4 months. Not all patients underwent chemotherapy because impairment of renal function or the patient refused. The ideal radiotherapy in management of local advanced stage cervical cancer is external radiation followed by brachytherapy. In our hospital, not all patients underwent brachytherapy after received external radiation because limitation of facility.Complete radiation in the form of ER 35x or ER 25x + AFL 2x. The selection of patients for this study was based on the medical records of patients indicating a diagnosis of stage III and IV cervical cancer who underwent radiotherapy in Dr. Soetomo Hospital. Monitoring of the patient's medical record within 5 years since being diagnosed with cervical cancer up to the 5 year time limit whether recurrences occured in patients to assess DFS. Patient’s outcome monitored within 5 years since being diagnosed with cervical cancer whether the patient is still alive or death to assess 5 years survival rate. The data is obtained by conducting interviews by phone, medical record data in the last time of control, or visits to the patient’s home if the number cannot connected. Data on clinical characteristics were collected and then analyzed to determine the association effect to disease free survival and 5 years survival.
Results and discussion
Total of 216 patients with stage III-IV cervical cancer after radiotherapy were included in the inclusion criteria and analyzed regarding variables that affect 5 years survival and disease free survival. The number of patients was obtained from medical record data and patient interviewed. Clinical data collection was obtained through medical records and followed up with telephone conversations. Outline of this study was divided into 3 major groups, there were clinical characteristics, limitations of radiotherapy, and patient outcomes in the form of 5 years survival and disease free survival. Clinical characteristics include age, histopathology, and tumor size. The limitations of radiotherapy include waiting list for radiotherapy, chemotherapy administration while waiting radiation, and the type of radiotherapy was given (Tables 1 and 2).
The number of patients who could be connected was less than 50% of the total study sample. It was because the number was inactive but the address was completed in the medical records were visited to the patient’s house. The average age of the research sample was 51.99 years with the youngest age being 29 years and the oldest age being 81 years. Mostly samples aged over 40 years (91.7%) and the most common histopatology is squamous cell carcinoma (74%). The average tumor size in this study sample is 5.45 cm, with the smallest tumor size being 4 cm and the largest being 12 cm. Mostly samples tumor size less than 6 cm (68.1%) (Tables 1 and 2).
The waiting list of radiotherapy in this study divided into 2 categories that were <4 months (29.2%) and >4 months (70.8%). The average waiting list is 4.53 months, with the lowest waiting list being 2 months and the longest being 12 months. There were 168 patients (77.8%) who underwent chemotherapy while waiting radiotherapy and 48 patients (22.2%) did not receive chemotherapy. Based on the therapy obtained, 48 patients (22.2%) were just ER, 117 patients (54.2%) underwent chemotherapy and ER, 51 patients (21.6%) underwent chemotherapy and ER + AFL, and none of the patients underwent just ER + AFL therapy. Overall, in the sample of patients diagnosed with cervical cancer in 2016-2017, the number of patients who died in 5 years was 177 patients (81.9%). The number of patients who experienced recurrences was 182 patients (84.3%) (Tables 1 and 2).
The results of the survival analysis showed that >40 years group had a higher life expectancy of around 24 months compared to <40 years group which was around 16 months (CI 95%, p-value 0.266) (Table 1). The results of the recurrence analysis showed that patients aged >40 years had DFS of around 19 months compared to the group <40 years which was around of 13 months (CI 95%, p-value 0.318) (Table 2).
A population-based retrospective analysis was conducted of 10,022 cervical cancer cases in England and Wales, the United Kingdom. The cohort approach showed significantly better overall survival among younger women (<40 years) when compared to cohort observations of older women (>40 years) [10]. Increasing age will result in decreased production of effector T lymphocytes, antibodies, and the ability to respond to foreign antigens including mutated cells. In young patients, it is estimated that the body's immune response has the ability to fight cell mutations that occur locally [11]. Studies have shown that older age is a significant risk factor for receiving less treatment or receiving no treatment at all compared to younger patients with the same diagnosis. This is because elderly patients have more comorbidities, so patients cannot receive standard therapy such as chemotherapy and radiotherapy which have many side effects, and thus reducing the patient's life expectancy [12]. The existence of insignificant results on the age factor may be due to the fact that in this study there were no restrictions on other significant factors, namely histopathology, tumor size, radiation time, and the received therapeutic treatment.
In this study, the results of the survival analysis showed that the squamous cell carcinoma, adenocarcinoma, and others groups had the same life expectancy, which was around 24 months (CI 95%, p-value 0.819) (Table 1). The results of recurrence analysis showed that the squamous cell carcinoma group had the highest DFS, which was around 21 while the adenocarcinoma group had the lowest, which was around 16 months (CI 95%, p-value 0.675) (Table 2).
The survival analysis's findings revealed that the group with tumor sizes less than 6 cm had a roughly 24-month life expectancy, while the group with tumor sizes larger than 6 cm had a roughly 21-month life expectancy (CI 95%, p-value 0.046) (Table 1). The recurrence analysis results indicated that the DFS for tumors smaller than 6 cm was approximately 22 months, while the DFS for tumors larger than 6 cm was approximately 12 months (CI 95%, p-value 0.037) (Table 2).
In previous study, the adenocarcinoma prognosis was worse than that of squamous cell carcinoma associated with a larger tumor (bulky), more resistance to radiotherapy, and a tendency to infiltrate intraperitoneal organs. Several studies have shown that adenosquamous carcinoma has a worse prognosis than adenocarcinoma with a higher recurrence rate and the prognosis is worse due to adenosquamous carcinoma mostly have a higher grade [13]. The histopathological type of adenocarcinoma is a significant and independent prognostic factor for disease free survival and local recurrence in advanced cervical carcinoma patients treated with radiotherapy. Advanced stage cervical carcinoma is also a significant and independent prognostic factor in local recurrences and metastases [14,15] The lack of limitations on other important factors in this study, such as age, tumor size, radiotherapy waiting time, and therapeutic treatment received, may account for the insignificance of the results regarding the histopathology factor.
Studies conducted by Sun et al., management of local advanced stage cervical cancer with a combination of CCRT and ER + AFL. This study evaluated 3 main parameters to assess the success of patient therapy. The 3 parameters were tumor size, tumor volume, and tumor volume reduction rate before and after CCRT. It was found that the 3 parameters had prognostic value on patient outcomes. With the rate of tumor volume reduction having the highest significant value [16]. Tumor size in cervical cancer is important since the microinvasive stage. The choice of therapy is based on the size of microinvasive carcinoma or truly invasive cervical carcinoma (>stage IB) when the tumor is larger than the microinvasive tumor. As size increases, so does the risk of nodular metastases, recurrence, lower survival rate, and failure of surgical treatment options and radiotherapy [15]. Larger tumor sizes also damage the normal structure of the cervical canal, making it difficult to apply brachytherapy. In this study show that tumor size have significant correlation with patient outcome. Patient with tumor size <6 cm had better survival rate and DFS than tumor size >6 cm (24 vs. 21 months and 22 vs. 12 months) [17].
Previous study was described that the tumor stage experienced a marked increase between 40 and 65 days. This study demonstrated reduced locoregional control and overall survival for patients who waited more than 40 days for radiation treatment. Patients with a radiation waiting list of more than 40 days had a 15% lower survival rate at 3 years of follow-up. Vaginal bleeding is a common clinical feature of cervical cancer. The longer patients wait for radiation treatment, the higher the chance they will need repeated blood transfusions or hemostatic brachytherapy to stop bleeding, thereby reducing the overall survival rate [18]. Study of Noh et al. showed that reducing the waiting time for CCRT could be one of the strategies to improve survival rate and disease free survival. A shorter waiting time from diagnosis to definitive CCRT resulted in good overall survival [19]. This study showed that waiting list radiotherapy have significant correlation with patient outcome. Patient with radiotherapy waiting list <4 months showed better survival rate and DFS than patients who waited >4 months (33 vs. 24 months and 24 vs. 16 months).
Based on survival analysis data, patients who received radiation therapy less than four months had a longer life expectancy (about 33 months) than those who received radiation therapy more than four months (about 24 months; CI 95%, p-value = 0.029) (Table 1). The group of patients who received radiation therapy less than four months had a higher disease-free survival (DFS) of approximately twenty-four months (CI 95%, p-value = 0.025) than the group receiving radiation therapy more than four months (Table 2).
The results of the survival analysis showed that the group of patients who received chemotherapy while waiting radiotherapy had a higher life expectancy, which was around 24 months compared to the group who didn’t, which was around 11 months (CI 95%, p-value <0.05) (Table 1).
The results of the recurrence analysis showed that the group of patients who received chemotherapy while waiting radiotherapy had a higher DFS which was around 22 months compared to the group who did not, which was around 10 months (CI 95%, p-value <0.05) (Table 2).
The group of patients who received chemotherapy plus ER AFL had the longest life expectancy, estimated at 44 months, according to the survival analysis results. This group outlived the chemotherapy + just ER group by about 24 months (CI 95%, p-value <0.05), and the just ER group by about 11 months (Table 1). According to the recurrence analysis results, the patients in the chemotherapy + ER AFL group had the longest DFS at approximately 34 months, while the patients in the just ER group only had a DFS of about 10 months, and the patients in the chemotherapy + just ER group had a DFS of about 16 months (CI 95%, p-value <0.05) (Table 2).
The result of the survival analysis showed OS the patient group with waiting list for radiotherapy <4 months with chemotherapy + ER AFL therapy was 35.7%. The results of the survival analysis showed that the group of patients with radiotherapy waiting list <4 months with chemotherapy and ER AFL had the highest life expectancy, which was around 50 months compared to other groups. Then the next waiting list group >4 months with chemotherapy therapy and ER AFL which is around 36 months and the lowest life expectancy in patients with radiation waiting time >4 months with just ER therapy was only about 10 months (CI 95%, p-value <0.05) (Table 3). The results of the recurrence analysis showed that the group of patients with radiotherapy waiting list <4 months with chemotherapy and ER AFL had the highest DFS of around 46 months when compared to other groups, and then the next waiting list group >4 months with chemotherapy and ER AFL which was around 34 months and the lowest is in patients with radiotherapy waiting list >4 months with just ER therapy only about 10 months (CI 95%, p-value <0.05) (Table 4).
The results of the univariate cox regression showed that tumor size (p-value= 0.045), radiation waiting list (p-value = 0.041), chemotherapy administration (p-value = 0.000), and type of radiotherapy (p-value = 0.000) had a significant p-value (<0.05) can be a prognostic factor for cervical cancer patient mortality for 60 months. Based on the hazard ratio value, tumor size >6 cm has a value of HR = 1.377, patients with radiotherapy waiting list >4 months had a value of HR = 1.422. Patients who did not receive chemotherapy while waiting radiotherapy had a HR = 2.602. Patients who underwent ER alone had a HR = 4.006 (Table 5).
Tumor size (p-value 0.048), radiation waiting list (p-value = 0.028), chemotherapy administration (p-value = 0.000), and type of radiotherapy (p-value = 0.000) all had significant effects (p-value <0.05), according to the univariate cox regression results. It indicates that during a period of 60 months, the following variables are predictive of recurrence in patients with cervical cancer: tumor size, radiotherapy waiting list, chemotherapy administration, and type of radiotherapy. Tumors larger than 6 cm had a hazard ratio value of 1.364, and patients on a radiotherapy waiting list longer than 4 months had a hazard ratio value of 1,448. The HR for those who did not receive treatment was 2.284. Individuals who received ER alone had an HR = 3.576 (Table 6).
Meta-analysis studies with more than 3,000 patients in randomized trials was not enough to explain the effectiveness of preradiation chemotherapy in the treatment of advanced cervical cancer. Although, the overall results of the meta-analysis do not support the use of platinum-based pre-radiation chemotherapy before radiotherapy for patients with advanced cervical cancer, good preparation is needed to determining the timing and intensive dose of pre-radiation chemotherapy as it may affect patient outcomes. There are two alternative preradiation strategies to improve longterm outcomes. One is the administration of chemotherapy with short cycles and intensive doses of platinum-based chemotherapy before radiotherapy, the second is similar chemotherapy given before surgery (with or without radiotherapy), is expected to provide a reasonable alternative to radical radiotherapy for early stage cervical cancer. However, in the case of advanced cervical cancer, it is still debated because in addition to insignificant overall survival and disease free survival outcomes, it also provides side effects from the administration of preradiation chemotherapy, reducing the feasibility of patients receiving radiation. Sardi et al. suggested that preradiation chemotherapy should be given intensively with radiotherapy at a distance of 7±14 days post radiation or surgery starting 4±6 weeks after chemotherapy. However, currently published results of concurrent chemoradiation still show better results [20,21].
Previous study shows that giving chemotherapy with short cycles and intensive doses of platinum-based chemotherapy before radiotherapy or giving similar chemotherapy given before surgery (with or without radiotherapy), is expected to provide an alternative to giving radical radiotherapy for cervical cancer. This is considered close to the results of the CCRT. Giving chemotherapy can inhibit tumor progression. Chemotherapy can cause radio-sensitization of tumor cells, resulting in a change in the shape of the cell survival curve after radiation. This is due to direct cytotoxicity to tumor cells or inhibition of repair of tumor cell damage due to sub-lethal or potentially lethal radiation [22]. This study showed that chemotherapy while waiting radiotherapy have significant correlation with patient outcome. Patients who received chemotherapy while waiting for radiotherapy had better survival rate and DFS than patients who did not (24 vs. 10 months and 22 vs. 10 months).
In general, all patients with locally advanced stage cervical cancer–Federation of Gynecology and Obstetrics (FIGO) stage IB2-IVA–should be considered for brachytherapy as part of definitive therapy. Based on the results of several randomized trials, CCRT has been the standard treatment for advanced cervical cancer since 1999. External beam radiotherapy (EBRT) followed by brachytherapy is associated with better outcomes than just EBRT. Administration of EBRT also has side effects including high levels of gastrointestinal toxicity and urogenital complications [22,23]. Brachytherapy is an important component of definitive therapy for locally advanced stage cervical cancer and is one of the most important treatment components for curing cervical cancer. The brachytherapy applicator delivers high doses to the cervix while limiting the dose to at-risk organs such as the rectum and bladder. This cannot be achieved by external beams alone because of the proximity of the organs at risk. This is why the effectiveness of brachytherapy for local tumor control is much better and can reduce the risk of side effects when compared to giving just external radiation [24-26]. In this study show that brachytherapy have significant correlation with patient outcome. Patients who got chemotherapy while waiting radiation and continued by ER AFL had better survival rate and DFS than patients who just received ER (44 vs. 24 months and 34 vs. 16 months). The difference in the type of radiotherapy given to patients is due to 3 factors where not all patients do not receive brachytherapy. These 3 factors include technical factors, patient factors, and facility factors. Technical factors were caused by difficulties in placing the brachytherapy applicator due to the large size of the tumor, vaginal atrophy, and the sonde could not enter the uterus. Patient factors because the patient was not cooperative, this is because ideally the implementation of brachytherapy under anesthesia. Facility factors due to limited radiation sources from brachytherapy.
Radiotherapy waiting list and type of radiotherapy are very significant in influencing the survival status of patients with radiation waiting time <4 months and received chemotherapy while waiting radiotherapy with a life expectancy of 48 months compared to radiation waiting time >4 months with only ER alone with a life expectancy only 10 months. If the patient underwent chemotherapy 4 times every 3 weeks and continued radiation before 4 months so that in accordance with the literature that the patient underwent sequential chemotherapy followed by radiotherapy with a range of 7-21 days which had outcomes close to concurrent chemoradiation. The explanation that radiation is carried out at least 7±14 days after chemotherapy administration is explained by the continued detection of platinum-based chemotherapy after 2 weeks of administration where it is still found that the chemotherapy is still working [21,27]. However, if range of chemotherapy to radiotherapy is too long, it can actually worsen the patient's outcome. This can be related to the work of cisplatin as a chemotherapy agent damaging DNA and eventually causing apoptis, but because it has a long enough waiting time for radiation to give time for the tumor to repair DNA damage which can efficiently cause chemoresistance in cancer cells [28].
Conclusion
Tumor size, radiotherapy waiting list, chemotherapy administration while waiting radiation, and type of radiotherapy are the most significant factors for outcome patient. In hospital condition with limited radiotherapy, sequential chemotherapy and continued by external beam radiotherapy and brachytherapy can be used as alternative therapy with the condition that the maximum period radiotherapy is given <21 days after the last chemotherapy administration if it is not possible to do concurrent chemoradiation.
Acknowledgments
The authors would like to express their gratitude to the instructors and supervisors who helped with the research supervision.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors have participated in this study in some capacity, including conception, interpretation, article drafting, and critical revision for significant intellectual content, and final approval
Conflict of Interest
The author declare no conflict of interest.
Orcid:
Dito Oktawijaya Pratama: https://www.orcid.org/0009-0003-8794-8223
Brahmana Askandar Tjokroprawiro: https://www.orcid.org/0000-0003-1658-3477
Lulus Handayani: https://www.orcid.org/0009-0005-8374-8253
---------------------------------------------------------------------------------------------------
How to cite this article: Dito Oktawijaya Pratama, Brahmana Askandar Tjokroprawiro*, Lulus Handayani, Correlation of clinic pathology characteristic with disease free survival and 5 years survival in stage iii-iv cervical cancer in low resource setting hospital. Eurasian Chemical Communications, 2024, 6(2), 223-236. Link: https://www.echemcom.com/article_183979.html
---------------------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
