Document Type : Review Article
Authors
1 Medical Biochemistry Department, Faculty of Medicine, Zagazig University, Egypt
2 Cardiology Department, Faculty of Medicine, Zagazig University, Egypt
3 Internal Medicine Department, Faculty of Medicine, Zagazig University, Egypt
Abstract
Coronary artery disease (CAD) refers to the progressive development of atherosclerosis, which is a global significant mortality cause, initial diagnosis of CAD is a challenge. Consequently, biomarkers are required for prediction of subclinical atherosclerosis and early treatment. Previously, miR-126 and miR-423-3p were approved to be participants in (CAD) development. Yet, their association with subclinical atherosclerosis is unidentified. The aim of our study is to investigate the role of plasma miRNA 126 and miRNA 423-3p levels to predict subclinical atherosclerosis and correlate it with exercise ECG test. The levels of miR-126 and 423-3p were assessed in plasma from 50 healthy control (group I) and 50 subclinical atherosclerotic CAD cases (group II) by quantitative real time –PCR (qRT-PCR). Exercise ECG was done to detect ischemic changes in both groups. There was a significant decrease of miRNA 126 and miR 423-3p levels in group II compared to group I. Likewise, a positive correlation was detected between both miR 126 and miR 423-3p with positive exercise ECG test. ROC curves of both miR 126 and miR-423-3p to detect positive exercise ECG test showed sensitivity and specificity of 92.2% and 93.9%, correspondingly. The combination of both miR-126 and miR-423-3p is considered as hopeful indicators of subclinical atherosclerosis in risky individuals, thus intensifying the management of hazard factors might decrease atherosclerotic progression.
Graphical Abstract
Keywords
Main Subjects
Introduction
Coronary artery disease (CAD) is a principal global health problem. Atherosclerosis and thrombosis causal CAD comprises many cell sorts [1]. Atherosclerosis is a long-lasting and advanced inflammatory disorder, which begins early in life and has an extended subclinical period. Numerous blood indicators concomitant with CAD were recognized, but only a limited number possess a diagnostic effect or imperative clinical inferences which influence managing plan [2].
Early anticipation of CAD is of proven impact aimed at the total decline of CVD fatality. Thus, indicators that can evaluate the hazard for CAD and the initial atherosclerosis development could be desired. Subclinical atherosclerosis is an early sign of atherosclerosis problem and its well-timed detection may decelerate development to CVD [3].
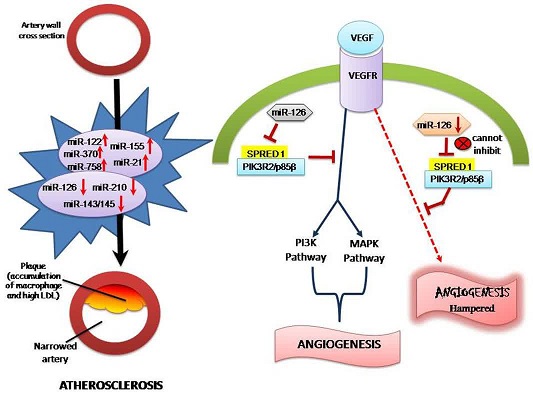
MicroRNAs (miRNAs) are non-coding RNAs that control the gene expressions tangled in several cellular procedures [4]. Vascular endothelium-enriched miR-126 was linked to CAD, atherosclerosis as well as the thrombosis occurrence. Remarkably, the miR 126 level has been detected to be augmented in ischemic heart and hypertensive patients [5,6]. Moreover, amplified levels of miR-126 are liberated from the platelets or endothelium into the plasma. As a result, they affect platelet motivation and coagulation via triggering the endothelial inflammatory response [5,7]. Increased level of miR-126 is detected in the endothelial inflammatory response through regulating expression of vascular cell adhesion molecule-1 (VCAM-1) [8]. Low levels of miR-126 summarize the antithrombotic rule of VCAM-1. Moreover, miR-126 level was described to be linked with the grade of heart failure [9]. Irrespective of therapy, miR-126 level was considerably up-regulated in multiple disorders of inflammation [10].
Various miRNAs (and particularly miR-126) reflect numerous characteristics of susceptible CAD, for instance hypoxia, inflammation besides extracellular matrix (ECM) ruin. Platelets or activated endothelial cells yield microparticles (MPs) that are liberated to the circulation of vulnerable CAD patients. These MPs contain miRNAs transferring them to their target cells [5-6,11].
Small dense LDL (sdLDL) is LDL particles which are 24.2-25.5 nm. Recently, an adding number of researches have demonstrated that sdLDL-C has a vital role in atherosclerosis (AS) development. However, no previous studies revealed its correlation with miR126 or miR423-3p [12].
Little data is available about the link between miR-423-3p and cardiac disease. Earlier studies showed that miR-423-3p was a regulator for TNF-α, signifying a potential effect in atherosclerosis. It was also stated that the exosomal miR-423-3p in the heart fibroblasts has cardio protective role in of ischaemic post-conditioning [13-14].
The aim of the current study is to investigate the role of plasma miRNA 126 and miRNA 423-3p levels to predict subclinical atherosclerotic CAD cases to target their therapy, via managing preventable risk factors aiming to decrease its burden in IHD development. It was hypothesized that plasma miRNA 126 and miRNA 423-3p levels are different in subclinical atherosclerotic CAD from controls, hence they can be used as biomarkers for its diagnosis. Our objectives included miR 126 and miR 423-3p levels assessment in case and control groups and their correlation with sdLDL.
Subjects and methods
Research subjects
Between January 2023 and May 2023, this case-control study was conducted at Zagazig College of Medicine Medical Biochemistry Department, Egypt. Fifty (50) subclinical atherosclerotic CAD cases were collected for the study at Cardiology Department. They had one or more risk factors for IHD (e.g., smoking, DM, hypertension, or family history for IHD). The cases were identified during routine checkup, showing ischemic changes on performing exercise ECG at the Cardiology Clinic. Fifty healthy individuals of the same age and sex participate as a control group. They had no risk factors for IHD and showed no ischemic changes on exercise ECG performing. A written consent was gained from all applicants beforehand the study. The study was implemented in accordance with Declaration of Helsinki. The study was approved by the IRB Committee of Zagazig College of Medicine with approval number (ZU-IRB # 10249/ 25-12-2022).
Each participant has performed exercise ECG was performed to find ischemic changes afterwards a thorough history taking that involved age, sex, family history, and other risk factors. We prudently examined and registered the values of fasting blood glucose, lipid report, and sd LDL.
Method
Acquisition of venous specimen
4 mL of venous blood specimens were collected on EDTA for real-time PCR investigation of plasma miRNA 126 and miRNA 423-3p values. Another 2 mL venous samples were obtained in plain tubes for estimation small dense LDL (sd LDL) to be correlated with miRNA levels. MiRNA extraction was through using miRNeasy kits from Qiagen, Germany. All of the steps were done according to the instructions of the kit.
cDNA production
The miRNA reverse transcription was performed by miScript IIRT kit Qiagen, and then the cDNA were relocated to a -20 °C freezer.
Amplification for miRNA Expression Levels
The amplification was made in a 20 µL mixture including 5 µL of the cDNA, 100 pmol/mL of each primer miRNA-126, miRNA 423-3p or RNU6 (internal control), 10 µL 2x QuantiTect SYBR Green PCR Master Mix and 4 µL distilled H2O. The amplification was carried out using Real time Cycler (Stratagene Mx3005P), as mentioned by the next procedure; the initial step at 95 ºC for fifteen min followed by 40 rounds of 95 ºC for fifteen second, 55 ºC for 30 second, and finally 70 ºC for 30 sec. The variation scale of the miRNA level detected in cases in comparison to controls was evaluated by the 2-ΔΔCt method.
Treadmill Exercise ECG Test
Treadmill exercise ECG test was executed to both groups according to Bruce protocol, to detect ischemic changes.
The sd LDL-C concentration was estimated from equation (in mg/dL) was sdLDL-C = 0.580 (non-HDL-C)+0.407 (d LDL-C)-0.719 (cLDL-C)-12.05 [15]. Direct LDL-C was measured using kits from DIALAB company, while calculated LDL was estimated from the equation (c LDL-C) (in mg/dL) = TC-(HDL-C)-(TG/5).
Results
Demographic characteristics and hazard factors among the studied groups was illustrated in (Table 2) and (Figure 1). Patients with atherosclerotic CAD cases (group II) exhibited higher significant prevalence of smoking (34 in group II vs. 0 in group I), DM (32 in group II Vs 0 in group I), HTN (41 in group II vs. 0 in group I) and family history (35 in group II vs. 0 in group I) compared to control group, while age and sex showed non-significant change. Regarding age the Mean± SD in group I was 48.78± 4.68 vs. 49.78± 5.2 in group II. Regarding sex, group I was 44% males Vs 56% females ,while group II was 46% males vs. 54% females.
The level of lipid profile including TC, TG, LDL, and small dense LDL was reported to be significantly higher in patients with atherosclerotic CAD cases (group II) compared to control group while HDL exhibited significant decrease in group II in comparison to control group. HDL mean ± SD was 150.87 ± 9.16 in group I vs. 193.74 ± 8.76 in group II (Table 3).
Positive Exercise ECG Test was significantly greater in patients with atherosclerotic CAD cases (group II) compared to control group. The mean miRNA 126 expression level in group I was 1.0 ± 0.06 whereas it was 0.24 ± 0.06 in group II. It was found that there has been significant decline of miRNA 126 expression level in patients with atherosclerotic CAD cases (group II) comparing to control group as shown in (Table 4).
The mean miRNA 423-3p expression level in group I was 1.01± 0.06 while it was 0.40± 0.03 in group II. It was found that there was statistically significant decrease in miRNA 423-3p expression level in patients with atherosclerotic CAD patients (group II) compared to control group, as presented in Table 4. A significant positive correlation was between mi-RNA 126 with positive exercise ECG test. In addition, there was significant positive relation between mi-RNA 423-3p with positive exercise ECG test. A substantial negative correlation was between mi-RNA 126 with cholesterol, triglyceride, LDL-c, fasting blood sugar, and small dense LDL (Figure 1). Meanwhile, a considerable positive correlation was between mi-RNA 126 and HDL, as summarized in Table 5.
There was significant negative correlation between mi-RNA 423-3p with cholesterol, triglyceride, LDL-c, fasting blood sugar, and small dense LDL as presented (Figure 2). Meanwhile, there is significant positive correlation between mi-RNA 423-3p with HDL, as presented in Table5.
The ROC analysis approved a good potential of miRNA-126 to detect positive ECG test at cut off 0.5 with sensitivity and specificity of 92.2% and 93.9%, respectively, as shown in Table 6 and Figure 3.
Also, miRNA-423-3p verified a good power to detect positive exercise ECG test at cut off 0.52 with sensitivity and specificity of 92% and 93.7%, respectively, as demonstrated in Table 6 and Figure 4.
Combination of both miRNA-126 and miRNA-423-5p giving nearly the same power to detect positive exercise ECG test with sensitivity and specificity of 93.9% and 93.8%, respectively, as demonstrated in Table 6 and Figure 5.
Discussion
Atherosclerotic cardiovascular diseases are considered as the main cause of mortality worldwide [16]. Atherosclerosis is a long-lasting disease, which develops in silent and asymptomatic manner. The clinical manifestations of CAD occur late after subclinical atherosclerosis initiation [17]. Thus, indicators that can assess the early atherosclerotic course and hazard for CAD occurrence is greatly required to reduce the burden of CVDs. These biomarkers could help in prediction and early pickup of ischemic patients among risky individuals for intensifying the management of their modifiable risk factors and early treatment.
Undoubtedly, the CAD risk is evaluated by algorithms dependent on patients’ hazard factors such as the American Heart Association or SCORE calculators [18-19]. However, those algorithms poorly assess an individual’s risk, particularly in young beings.
One approach for the prediction of cardiovascular disease risk is ECG exercise test that is already used in clinical practice and it is a basis to diagnose cardiac ischemia in risk groups and has the gain of being non-invasive and safe. American Heart Association guidelines praise the exercise ECG test as the first indicative check for ischemia in moderate risky patients who are capable of exercise and possess interpretable resting ECG graphs [20]. Our work revealed that, Positive Exercise ECG Test was significantly elevated in patients with atherosclerotic CAD cases compared to control group. However, the Exercise ECG Test has different sensitivity and specificity in various studies and has its own restrictions as it is a subjective study and cannot be afforded by all patients due to orthopedic problems [21]. Therefore, there is a necessity for more straightforward, less time-wasting, and easy to be surveyed in outpatient clinics and operator-independent indicators that can correlate with ECG exercise test.
Singh T et al. suggested that atypical results of exercise ECG possessed a strong association with CAD mortality or myocardial infarction in comparison to normal conclusions of exercise ECG [22].
Another tactic to overcome these limits is the use of microRNAs (miRs) as novel biomarkers for precise cardiovascular hazard anticipation and early dealing of CAD as they are simple, non-invasive, and sensitive. MiRNAs are well-known to be main players in moderating the function of endothelial cells as well as macrophages, modifying atherosclerosis development [23-24]. They are supreme indicators as they are fairly tissue-specific and persist stable in the circulation [25-26 ].
MiR 126 has a vital controller role in vascular integrity and homeostasis [27-28]. It is definitely and greatly expressed in the endothelial cells, (ECs), that regulates atherosclerosis mechanisms [29]. In the present work, it was theorized that the circulating miRNA 126 levels can expect the occurrence of significant CAD. It was found that, there was statistically substantial decline of miRNA 126 expression levels in patients with subclinical atherosclerotic CAD cases compared to control group. The specificity and sensitivity of miR-126 were evaluated to assess the feasibility of using it as the predictor of atherosclerosis help in improvement of CAD patient management in clinical practice. Our findings showed also a significant negative correlation between mi-RNA 126 with cholesterol, triglyceride, LDL-c, fasting blood sugar and small dense LDL. Meanwhile, there was substantial positive association between mi-RNA 126 with HDL.
Our findings are consistent with previous study by Wang et al. who detected that miRNA-126 expression was decreased in CAD patients. They observed that the miRNA126 expression in plasma was connected to the severity of CAD [30]. Our results were consistent with those of Mahendra et al., who found reduced expression of micro-RNA-126 in patients with CAD when compared to control group [31]. The same was documented by study performed by Zang et al., about atherosclerosis in hypothyroid cases [32]. It was concluded that miRNA-126 has a role in assessment of the hazard for CAD and initial atherosclerosis [33-34].
The angiogenic action of miR-126 is intermediated by stimulating MAP kinase and PI3K signaling as a result of released VEGF [34-36]. Harris et al. observed that miR-126 impedes vascular cell adhesion molecule-1 synthesis, which is incorporated in leukocyte connection to endothelium [37]. In addition, miR126 was related to serum lipids in a study by sun et al. [38].
Due to the important association between miR-126 level and LDL cholesterol, the plasma miRNA values may tell a compensating reaction to inflammation. MiRNA-126 keeps the vascular integrity. It has a double role in the atherosclerosis development decreasing VCAM-1 expression; thus, restraining the leucocyte adherence to form the plaque. Though, it as well increases smooth muscle cell multiplying and stimulation that may amplify the atherosclerosis development [39].
Conflicting results also exist, which presented an increase of miR-126 level in myocardial infarction [40-41]. Conversely, in an another study, there was no significant change in miR-126 level in CAD patients, but in reverse correlated with LDL- cholesterol, providing vision into the possible role of miR-126 in metabolism of cholesterol [38].
MiR-423-3p, formerly well-thought-out to be an oncogene in different types of cancers, as well recognized as a lung cancer indicator [42]. In the current work, we found significant decrease in plasma miRNA 423-3p expression level in patients with subclinical atherosclerotic CAD cases compared to control group.
Our findings were in accordance with the data recorded by the major research so far assessing the prognostic role of plasma miRNAs in a general Chinese population, a significant decrease in miR 423-3p in CAD cases in comparison to controls. It was proved that the miR-423-3p would considerably increase the prediction of CAD depending on standard cardiovascular hazard factors [43].
Interestingly, the data collected by Rizzacasa et al., signifying a variance in expression of miR-423-3p, between stable and unstable CAD cases, so directing to the role of miR-423-3p as a potential biomarker for the assessing of MI hazard [44]. It was proposed that miR-423-5p has pro-apoptotic role of in cardiac cells. Hence, miR-423-3p down-expression in circulation in the first day after the MI incidence, could be reflected as a “cardiomyocytes mirror” reflecting a “reduction of cardiac cells apoptosis as well to enhance heart recovery during first phases of AMI [44].
However, Nabialek et al. observed a significant increase of miR-423 in MI patients in comparison with controls, meanwhile no substantial variances in miR-423 level were noticed at the follow-ups. Consequently, they show miR-423 as an initial biomarker of myocardial infarction [45].
In previous studies, they found miR-423-3p was noticeably downregulated by TNF-α, signifying a potential role of this miRNA in EC stimulation, the serious phase of atherosclerotic process. Important data showed that Cardiac fibroblast (CF) has cardioprotective effect by increasing the expression of exosomal miR-423-3p, which decrease the expression of its target gene RAP2C, which in turn increase the viability and decrease apoptosis [13-14].
Our study revealed a significant positive correlation between mi-RNA 126 with positive exercise ECG test .In addition, there was significant positive relation between mi-RNA 423-3p with positive exercise ECG test.
Furthermore, the combination of miR-126 and miR-423-3p was a significant biomarker for the evaluating of CAD, possessing a real diagnostic power. Collectively, they might possess a more valuable diagnostic or prognostic importance than any lone miR. Upcoming follow-up research is required to confirm their clinical significance.
Conclusion
In this study, we concluded that miR-126 and miR-423-3p could be promising biomarkers for risk prediction of subclinical atherosclerosis. Also, plasma miR-126 and miR 423-3p showed significant correlation with TG, TC, HDL, and LDL, suggesting they may influence the occurrence of atherosclerosis through these mechanisms. Further researches should highlight the follow up of these patients to show the correlation of these miRs in diagnosis and prognosis CAD.
List of abbreviations
IRF-1: Interferon regulatory transcription factor 1
VCAM-1: Vascular cell adhesion molecule-1
Sd-LDL: Small dense low density lipoprotein
Acknowledgements
None.
Orcid:
Heba Fawzy*: https://orcid.org/0000-0002-9497-1662
Doaa M. Hendawy: https://orcid.org/0000-0002-4791-8375
Hend Sameh: https://orcid.org/0009-0007-1347-9398
Hala Mosaad: https://orcid.org/0000-0002-4423-2503
---------------------------------------------------------------------------
How to cite this article: Heba Fawzy*, Doaa M. Hendawy, Mohammed S.Gharee, Mahmoud Amer, Hend Sameh, Hala Mosaad, The value of plasma mir 126 and miR 423-3p levels in prediction of subclinical atherosclerotic coronary artery disease. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(5), 609-622. Link: https://jmpcr.samipubco.com/article_187607.html
---------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
