Document Type : Original Research Article
Authors
- Petrina Theda Philothra 1, 2
- Andriati Andriati 1, 2
- Imam Subadi 1, 3
- Indrayuni Lukitra Wardhani 3
- Abdul Jabbar Al-Hayyan 1, 4
- Amandha Boy Timor 1, 2
- Mohammad Zain Chilmi 1, 4
- Soenartalina Melaniani 1, 5
1 Faculty of Medicine, Airlangga University, Surabaya, Indonesia, 60264
2 Department of Physical Medicine and Rehabilitation, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia, 60264
3 Department of Physical Medicine and Rehabilitation, Airlangga Teaching Hospital, Surabaya, Indonesia, 60264
4 Department of Orthopaedic and Traumatology, Dr Soetomo General Academic Hospital, Faculty of Medicine Airlangga University, Surabaya, Indonesia, 60264
5 Department of Epidemiology, Biostatistics, Population Studies, and Health Promotion, Faculty of Public Health, Airlangga University, Surabaya, Indonesia, 60264
Abstract
Osteoarthritis (OA) is the leading musculoskeletal disease that disrupting quality of life. ESWT has been regarded as promising noninvasive OA management. This study aims to assess how ESWT affects OA patient’s cartilage degradation using urinary C-terminal crosslinking telopeptide of type II collagen (uCTX-II) biomarker. Thirteen OA patients were selected in this one-group pretest-posttest design. Each participant received ESWT intervention once a week for six weeks. Piezo Shockwave 2 (focused-ESWT) machine with an intensity 0.27 mJ/mm2, frequency 4 mHz, and total impulse 4000 shocks was used. Cartilage degradation was evaluated by uCTX-II before therapy (T1), at three weeks (T2) and six weeks after completing serial intervention (T3). uCTX-II results improved significantly during T2 (p=0.000) and got the peak at T3 compared to before treatment (p=0.001). Repeated measure test using general linier model demonstrated significant gradual pain reduction (p=0.00). Pearson correlation test revealed that pain scale (WBS) was highly associated with uCTX-II baseline result (p=0.002, r=0.781). ESWT is an effective and safe intervention allowing knee pain reduction and improvement in OA cartilage degradation.
Graphical Abstract
Keywords
Introduction
Osteoarthritis (OA), the most prevalent type of arthritis, is a degenerative joint disease that recognized as the 11th leading cause of worldwide disability and poor quality of life [1,2]. It is reaching 53% in Southeast Asia [3]. Articular cartilage degradation, subchondral sclerosis, osteophyte formation, and bone remodeling are the main manifestations of OA [2,4]. Physical modalities, exercise, and medicamentosa are still the most often prescribed approaches due to non-invasive and have lower risk of complications [5,6]. However, the conventional therapeutic concepts more focus on symptom relief than regenerative effects that reserve the destruction of joint surface despite of the systemic adverse events from medicamentosa [1,2,5].
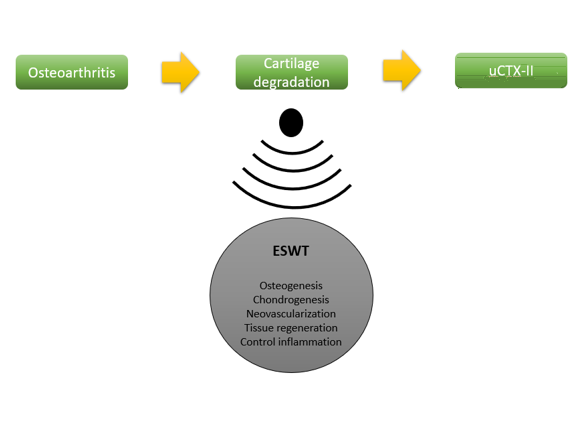
ESWT has gained interest recently as a potential therapy for OA [7]. It is a non-invasive method for managing OA providing targeted cell modification employing nanotechnology as regenerative medicine that created by piezoelectric generators [8]. The majority of research mostly looked at how well therapy improved functional indicators, while the study for biomarker of joint cartilage has never been studied. Quantifying the total dose of ESWT is also critical in reducing knee OA symptoms and restore cartilage degeneration [9].
Biomarkers are mostly employed in research nowadays in order to recognize diseases in early stages, disease progression, and facilitate the innovative treatments. Several markers in OA categories are broken down into synovial tissue production (anabolic activity), synovial tissue destruction (catabolic activity), and cartilage synthesis [10]. Since cartilage degradation is the primary pathology mechanism in OA, it has been primarily employed as a biomarker [11]. uCTX-II is the most effective cartilage degradation biomarker in terms of disease severity, prognosis, and has association with OA severity assessed by radiological examination [12]. In addition, it is important to figure out the correlation between uCTX-II with the patient’s functional circumstances, especially the pain scale as the main symptom of knee OA.
The uCTX-II selection is based on its specific relevance to cartilage degradation. uCTX-II is a fragment released during the breakdown of type II collagen, a major component of articular cartilage. As such, uCTX-II levels in urine provide a direct reflection of cartilage turnover and degradation, making it a specific and sensitive biomarker for assessing changes in joint health, particularly in the context of OA progression [13]. The choice of uCTX-II aligns with the goal of capturing changes in cartilage integrity, which is a key aspect of the impact of ESWT in OA patients.
While serum high-sensitive C-reactive protein (hsCRP) is a widely used biomarker for systemic inflammation and is associated with various inflammatory conditions, including arthritis, its direct connection to cartilage degradation is less specific than uCTX-II. hsCRP is a general marker of inflammation and might not provide the same level of precision in assessing the localized changes occurring in cartilage tissue [14]. Since the primary focus of our study is on the specific effects of ESWT on cartilage, uCTX-II is deemed more suitable for capturing changes in the targeted tissue.
The purpose of this study is to determine whether ESWT can decrease the degeneration of joint cartilage using uCTX-II. The second aim is to examine the potential correlation between uCTX-II and pain reduction as well as the different effects of serial frequency on pain reduction and uCTX-II results during both T2 and T3 evaluation.
Subjects
Ethical review approval was provided by the Ethical Committee of Soetomo General Academic Hospital Surabaya. The time enrollment was from May to August 2022. We used a simple random sampling technique. All participants were provided with information about the study’s purposes and signed informed consent. Thirteen knee OA patients who were receiving therapy at Physical Medicine and Rehabilitation (PMR) in Soetomo General Hospital met the inclusion criteria. To participate, each patient had to accomplish the following inclusion requirements as follows: 1) knee OA patient grade II-III according to K-L classification, 2) aged 50 years or older, 3) be able to walk independently with or without assistive devices, 4) no cognitive impairment (Moca-INA ≥26), 5) and no neurological impairment, 6) no ROM restriction, deformity and angulation impairment in lower extremities, and 7) be able to participate. We excluded patients with these following conditions: 1) autoimmune disease and or secondary arthritis, 2) history of surgery in knee joint and or intraarticular injection within the last 6 months, 3) blood clotting disorder and or currently on medication that manifests in blood clotting disorder, 4) malignancy in bone and or treatment area, 5) pregnancy, 6) lesion in treatment area, 7) hypersensitivity to ESWT and or coupling gel, 8) recently knee trauma, and 9) uncontrolled Diabetes Mellitus. Patients would drop out if they did not attend any serial treatment program, refused to continue the program, getting sick, and passed away.
Methods
This is a pre-experimental study using one-group pre-test post-test design. We used focused-ESWT Piezo Shockwave (Elvation®, Germany) for treatment with low to moderate intensity that increased gradually in each treatment session (0.04-0.27 mJ/mm2) following starts low and goes slow principle with Energy Flux Density (EFD) F10/G4, frequency 4 mHz, total impulse 4000 shocks within total 6 sessions once a week. Subjects were placed in supine position with the affected knee flexed 60-90 to expose the joint cartilage. The physician located pain area as the focus region that will receive greater therapeutic effects and avoiding main nerves and blood vessels area. Neither topical analgesic nor local anesthesia was used. Clinical signs and symptoms, WBS, and vital signs were evaluated during T1, T2, and T3.
uCTX-II as a catabolic marker of collagen type II is excreted in synovial fluid and urine. Every subject collected mid-stream morning void urine aseptically into the sterile tube. It was centrifuged at 2000-3000 rpm for 20 minutes. Human uCTX-II ELISA kit marker (Elabscience Biotechnology Laboratory Inc, Shanghai, China, catalog number E-EL-H0837) was evaluated using an enzyme-linked immunoassay (ELISA) procedure.
Outcomes
The primary outcome of this study was the changes of uCTX-II result as biomarkers of cartilage degradation. It was assessed at T1, T2, and T3. Secondary outcome was pain scale changes, assessed by WBS score that was evaluated every week. The correlation between uCTX-II and pain scale result was also evaluated.
Statistical analysis
This study data was analyzed using IBM SPSS statistics version 26. Saphiro-wilk test was used as normality test. Paired t-test evaluated the effect of ESWT intervention in uCTX-II results which followed by effect size evaluation using Cohen’s d. P <0.05 was regarded significant. Pearson’s correlation was an additional analysis to evaluate the correlation between subject characteristics with uCTX-II result. Improvement of pain scale (WBS) evaluated by repeated measure from general linear model test.
Results
All subject characteristics and variables were normally distributed. Subject predominant characteristics including aged 50 years or older, female gender, and obese were all associated as the risk factors of knee OA (Table 1) [16]. Paired-t test showed significant effects in T2 compared to T1 and T3 compared to T2 as well (Figure 1) which revealed significant effect size shown by Cohen’s d test (Table 2). Pain scale evaluation using repeated measures from the general linear model test showed p-value 0.00 (Figure 2). Pearson’s correlation revealed significant correlation (p=0.002) between pain scale (WBS) and uCTX-II result (r=0.781).
Discussion
This is the first study evaluating the effect of ESWT with a determined dose in cartilage degradation improvement which is shown by uCTX-II (Figure 3). uCTX-II correlates with pain scale and functional scale like WOMAC questionnaire [12]. In OA, the collagen equilibrium will be altered due to proinflammatory cytokine and protease that increases cartilage degradation, Matrix Metalloproteinase (MMP) and cell differentiation lead to hypertrophy and replacement of collagen type II with collagen-X. In spite of that, chondrocyte expression will vary due to chondrocyte aging and apoptosis [15]. It will gradually lose cartilage; enhance osteophyte production in subchondral bone, and cause fibrosis and vascular changes in synovial membrane [16-17]. This collagen type II is then subsequently broken down by degradation enzymes and excreted in urine [18-19].
The improvement in all subject’s uCTX-II might be related to neovascularization, osteogenesis, chondrogenesis processes, tissue regeneration, and improvement of inflammatory response [1-2,6]. The therapeutic dosage in this study was related to the previous systematic review using focused-ESWT with the frequency 3 to 8 weeks, 1-2 times per week, total 2000 to 4000 impulses, and moderate intensity 0.03-0.4 mJ/mm2 [1,2]. No serious adverse event was found in all subjects. Only one subject experienced mild discomfort that relieved in less than 24 hours after utilizing ice and taking NSAID. It was correlated with prior study that demonstrated no neuromuscular or systemic problems following therapy [18]. Due to the effectiveness, moderate intensity (EFD 0.04 mJ/mm2) is more recommended than mild intensity (EFD 0.093 mJ/mm2). In comparison to moderate intensity, higher intensity (EFD >0.5 mJ/mm2) causes more discomfort, swelling and local inflammation [21].
Pearson analysis revealed association between uCTX-II results and WBS (r=0.781). It indicates that the more severe pain, the higher uCTX-II result would be, and it is related to the greater cartilage breakdown within the joint cartilage [22]. Previous study from Bihlet (2019) found an association between pain and the degree of osteoarthritis. The differences of uCTX-II level was representative with pain symptom as manifestation of pathophysiology process of OA [14]. It demonstrates relationship between the rate of cartilage deterioration and joint lubricant changes, inflammation process, and lubricant loss [16,23].
Repeated measures analysis from general linear model revealed significant improvement in WBS within the serial number of ESWT therapy. The previous study showed improvement in pain scale of OA patients after 2 weeks of ESWT therapy, although there was no discernible difference with the control group. It demonstrates the significance of ESWT frequency to make an adequate change for patient’s symptom [8]. The pain relief induced by ESWT is comparable to that brought on by hyaluronic acid injection. ESWT had a long-term benefit in lowering pain at 6 and 12 months of evaluation [8,20].
ESWT (Extracorporeal Shock Wave Therapy) has several pain-relieving mechanisms, including the selective dysfunction of unmyelinated sensory fibers. This leads to a decrease in neuropeptides, specifically calcitonin gene-related peptides, within the dorsal root ganglia [21]. In addition to neovascularization and tissue regeneration, physical pressure of ESWT triggers biological mechanisms that are related to inflammation regulation process, and chondrogenesis improvement [1,2]. Furthermore, it reduces chondrocyte apoptosis and nitric oxide level in synovial cavity [23]. It occurs as a result of better control of growth factors regulation, angiogenesis, and neovascularization-promoting factors including endothelial nitric oxide synthase (e-NOS) and Vessel Endothelial Growth Factors (VEGF). In osteoblast, it is also linked to osteogenesis transcription factors such as VEGF-A and Hypoxia Inducible Factor-1-α. It boosts BMP-2, PKB, and TGF-β1 in facilitating osteoblast proliferation and differentiation [34]. Osteogenesis induced by an increase of calcium signaling caused by expression of Pdia-3, a crucial component of 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) and gene transcription. Moreover, it also enhances Mesenchymal Stem Cells (MSCs) metabolism promoting bone regeneration and chondrogenesis [2,23].
Limitation
We recognize several limitations in this pre-experimental study. We did not include control patients and merely perform moderate follow up of ESWT treatment. Hopefully, the following study will assess the impact of ESWT therapy over a longer duration with a control group.
In Figure 3, x-axis denotes the treatment sessions, indicating the sequential application of ESWT, while the y-axis represents the quantified levels of uCTX-II. Notably, the graph highlights trends such as an initial baseline, potential fluctuations during the early sessions, and a discernible trend towards decreased uCTX-II levels as the treatment progresses.
The significance of this information lies in its ability to demonstrate the impact of ESWT on cartilage health by observing changes in the uCTX-II biomarker. A declining trend in uCTX-II levels may suggest a positive response to ESWT, indicating reduced cartilage degradation and improved joint health over the treatment period. These insights contribute valuable data to our understanding of the therapeutic efficacy of ESWT in managing cartilage deterioration in OA patients.
Conclusion
After conducting three successive evaluations, the intervention involving Extracorporeal Shock Wave Therapy (ESWT) resulted in an improvement in cartilage deterioration among patients with osteoarthritis (OA). The positive changes became noticeable during the mid-test evaluation and reached their maximum effectiveness after six consecutive treatments. The improvement measurement was based on the uCTX-II biomarker.
In summary, the conclusions suggest that ESWT had a positive impact on cartilage deterioration in patients with osteoarthritis. The improvement was observed during the mid-test evaluation and reached its peak after six consecutive treatments, as measured by the uCTX-II biomarker.
Acknowledgements
None.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Conceptualization, data analysis, and drafting: Petrina Theda Philothra, Amanda Boy Timor. Review and revising: Andriati, Imam Subadi, Indrayuni Lukitra Wardhani, Abdul Jabbar Al-Hayyan, Mohammad Zain Chilmi, Soenartalina Melainani
Conflicts of interest
No potential conflict of interest relevant to this article was reported by the authors.
Orcid:
Petrina Theda Philothra: https://orcid.org/0009-0007-7023-6851
Andriati: https://orcid.org/0000-0002-1907-9551
Imam Subadi: https://orcid.org/0000-0003-3660-2583
Indrayuni Lukitra Wardhani: https://orcid.org/0000-0002-9889-9039
Abdul Jabbar Al-Hayyan: https://orcid.org/0009-0004-3143-1994
Amandha Boy Timor: https://orcid.org/0000-0001-9739-2968
Mohammad Zain Chilmi: https://orcid.org/0000-0002-1367-5256
Soenarnatalina Melaniani: https://orcid.org/0000-0002-4449-153X
-------------------------------------------------------------------------------------
How to cite this article: Petrina Theda Philothra, Andriati Andriati*, Imam Subadi, Indrayuni Lukitra Wardhani, Abdul Jabbar Al-Hayyan, Amandha Boy Timor, Mohammad Zain Chilmi, Soenartalina Melaniani, Regenerative effect of extracorporeal shockwave therapy in knee osteoarthritis patient. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(5), 665-674. Link: https://jmpcr.samipubco.com/article_187674.html
-------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
