Document Type : Original Research Article
Authors
1 College of Medicine, University of Al-Ameed, Karbala, Iraq
2 College of Medicine, University of Babylon, Babil, Iraq
3 Energy and Renewable Energies Technology Center, University of Technology, Baghdad, Iraq
Abstract
COVID-19 patients exhibit a diverse range of symptoms, with some individuals remaining entirely asymptomatic. Certain patients experience minimal or no noticeable symptoms, and in some cases, oxygen levels significantly drop without prominent clinical manifestations, a phenomenon is known as "silent" or "happy" hypoxia, which can potentially pose substantial risks to patients. A clinical study aims to assess the significance of silent hypoxia in COVID-19 patients' clinical course and to predict risk factors associated with it. 85 confirmed COVID-19 patients with hypoxia were included in the study and exhibited room air oxygen saturation (SPO2) levels below 92%. Comprehensive patient histories and physical examinations were conducted, with the severity of breathlessness evaluated using a scale ranging from 0 to 10. Breathlessness scored below 5 was categorized as silent hypoxia. Various laboratory tests, including lymphocyte counts and serum ferritin levels, were performed. The findings of our investigation indicate that a majority of the patients in our study demonstrated favourable outcomes. Notably, no significant disparity was observed in terms of clinical outcomes between hypoxic and non-hypoxic COVID-19 patients (p>0.05). Risk factors identified including elevated body mass index (BMI), age, and increased respiratory rate (RR) are addressed in this work and diagnosed as risk factors. Silent hypoxia is a phenomenon observed among COVID-19 patients, yet it does not appear to impact patients' clinical trajectories significantly. Obesity emerges as a potential risk factor for severe disease. High BMI and elevated RR are recognized as potential risk factors associated with the occurrence of silent hypoxia.
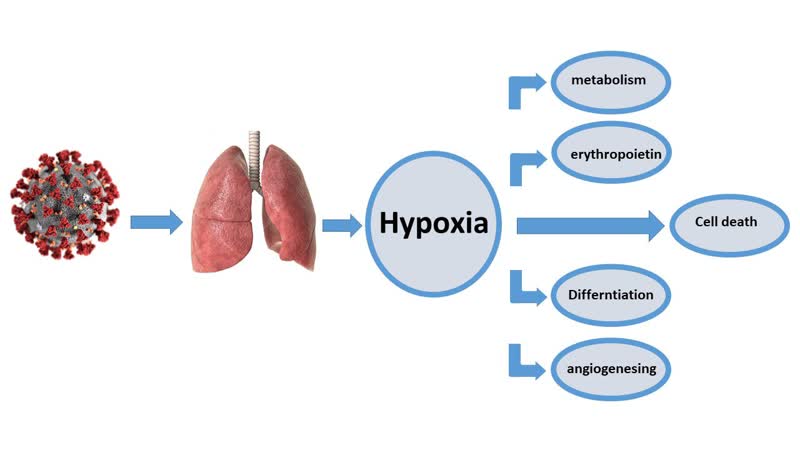
Graphical Abstract
Keywords
Main Subjects
Introduction
The virus belongs to the Coronaviridae family within the Nidovirales order, characterized by a diameter ranging from 65 to 125 nm and containing single-stranded RNA as its genetic material [1-3]. Initially termed the 2019 novel coronavirus (2019-nCoV), this newly identified pathogen emerged in Wuhan. It was later officially named by the International Committee on Taxonomy of Viruses (ICTV) as SARS-CoV-2, causing the disease termed COVID-19 [4-8].
As of the current writing, the novel coronavirus has affected around 120,000 individuals across 109 countries, with a mortality rate of 2.9%. The transmission rate of SARS-CoV-2 appears to surpass that of its predecessor, SARS-CoV, possibly attributed to genetic recombination events within the S protein's receptor-binding domain (RBD) [9].
COVID-19-infected patients commonly display symptoms of pneumonia accompanied by widespread alveolar damage, often progressing to acute respiratory distress syndrome (ARDS) [9-11]. In the context of the COVID-19 pandemic, the phenomenon of silent hypoxemia, characterized by substantial oxygen deficiency without noticeable breathlessness, has gained attention. This state is associated with shunt physiology, preserved lung compliance, and minimal dead space [9,12,13].
It encompasses lung pathologies resulting in localized shunting while sparing the remainder of the lung, such as lobar pneumonia or atelectasis. Silent hypoxemia can persist, occasionally prompting affected individuals to seek medical attention. COVID-19-related lung injury involves viral-induced inflammation, dysfunction of alveolar epithelia, alveolar collapse, impaired surfactant function, and heightened pulmonary artery pressure [14-16].
Although the condition has been classified as acute respiratory distress syndrome (ARDS), it deviates from the typical ARDS presentation. Notably, patients may exhibit normal lung compliance and lack overt respiratory distress despite hypoxemia [15]. Patients frequently arrive at medical facilities in a stable condition, conversing comfortably, despite oxygen saturation levels as low as 80%, thus highlighting the potential of pulse oximetry as a marker of respiratory distress [14,15,17].
Key characteristics of silent hypoxemia include intra-pulmonary shunting, maintained lung compliance, limited excessive dead space, absence of substantial pulmonary embolism or microvascular thrombosis, and dysfunctional hypoxemic vasoconstriction [14,15].
COVID-19 patients experience a gradual onset of severe hypoxemia alongside hypocapnia. Elderly age, obesity, atelectasis, and pre-existing structural lung disorders are potential predisposing factors for rapid clinical deterioration [14-15,18]. Unlike traditional tachypnea-associated hypoxemia, COVID-19-related hypoxemia does not typically lead to subjective breathlessness; instead, patients exhibit enhanced respiratory drive and minute ventilation. Consequently, augmented carbon dioxide (CO2) clearance prevents a sense of breathlessness [14,15].
Silent hypoxemia is categorized into stages known as subclinical, detection, and high mortality zones. The subclinical zone features normal oxygen saturation and respiratory rate. In the detection zone, oxygen saturation gradually declines, without subjective breathlessness, while respiratory rate begins to rise, indicating progressive self-inflicted lung injury (SILI). This zone necessitates intervention, as initiating treatment can yield dramatic outcomes. The high mortality zone is characterized by decreasing oxygen saturation, heightened breathlessness, and potential respiratory failure [14,15].
Silent hypoxemia can transform into a severe form when supplemental oxygen fails to yield significant improvement [19,20].
Hypoxemia primarily arises due to ventilation-perfusion mismatch, resulting in poorly oxygenated blood from the right ventricle entering the left ventricle [21]. Adjusting inhaled oxygen concentration can address this issue. In addition, shunt physiology contributes to desaturation in cases of anatomical abnormalities or non-functioning lung areas [14,15,19,20]. Patients showing desaturation despite high supplemental oxygen often have a notable shunt. CO2 clearance depends on effective gas exchange and is influenced by factors like dead space in conditions such as ARDS or pulmonary embolism, where gas exchange occurs without CO2 clearance [14,15,19].
Increased dead space necessitates greater gas inhalation for maintaining adequate CO2 levels. Silent hypoxemia alongside normal breathing arises from right-to-left shunting, regular lung compliance and resistance, and minimal excessive dead space [19,20]. This study aims to assess the clinical significance of silent hypoxia in COVID-19 patients and to identify potential risk factors associated with its development
Study objectives
The main objective of the study is to examine the prevalence and characteristics of silent hypoxia in COVID-19 patients, analyze the correlation between silent hypoxia and clinical outcomes in individuals with COVID-19, identify potential risk factors, such as age, obesity, and underlying lung conditions, that contribute to the occurrence of silent hypoxia, explore the relationship between silent hypoxia and respiratory parameters, such as respiratory rate and lung compliance, propose interventions and strategies for detecting and managing silent hypoxia in COVID-19 patients, particularly during the subclinical and detection stages, and contribute to a better understanding of the pathophysiological mechanisms underlying silent hypoxia in the context of COVID-19 and its implications for patient care.
Novelty of the study
This study brings four several novel aspects in the context of silent hypoxia among COVID-19 patients:
Clinical significance analysis
The study focuses on comprehensively evaluating the clinical significance of silent hypoxia in the context of COVID-19. This involves investigating its prevalence, impact on patient outcomes, and potential interventions.
Risk factor identification
The study seeks to identify specific risk factors associated with the development of silent hypoxia in COVID-19 patients. By exploring factors like age, obesity, and underlying lung conditions, the research aims to enhance our understanding of the predisposing elements to this phenomenon.
Respiratory parameters investigation
This research examines the relationship between silent hypoxia and respiratory parameters, such as respiratory rate and lung compliance. This analysis provides insights into how these factors interact and contribute to the manifestation of silent hypoxia.
Intervention strategies
The study endeavors to propose effective strategies for detecting and managing silent hypoxia, particularly during the subclinical and detection stages. This aspect of the research contributes practical insights into improving patient care and outcomes.
Pathophysiological insights
By exploring the pathophysiological mechanisms underlying silent hypoxia in COVID-19 patients, the study contributes novel insights into the complex interactions between lung function, oxygenation, and clinical presentation. Overall, the unique combination of clinical significance assessment, risk factor identification, respiratory parameter exploration, intervention strategies, and pathophysiological insights sets this study apart and offers novel perspectives on the phenomenon of silent hypoxia in the context of COVID-19.
Study design and participants
This multi-center cohort study was conducted in Babylon, Iraq, spanning from February to September 2020, specifically at Merjan Medical City. The study aimed to analyze and understand the phenomenon of silent hypoxia in COVID-19 patients. The cohort consisted of 85 patients who were admitted to the hospital due to COVID-19.
Inclusion criteria
Confirmed diagnosis
Patients were included if they tested positive for COVID-19 via PCR or exhibited classic COVID-19 findings on CT scans, as outlined by the British Radiology Society. Oxygen Saturation: Patients were required to have a baseline oxygen saturation (SPO2) level of less than 92% while breathing room air.
Data collection
For each patient, a comprehensive set of data was collected, including socio-demographic features, clinical assessments, radiological findings, and laboratory results.
Socio-demographic features
Key factors such as age, gender, weight, height, body mass index (BMI), and smoking history were recorded.
Assessment of breathlessness
The degree of breathlessness was evaluated using a scoring scale ranging from 0 to 10, where a higher score indicated more severe shortness of breath. Patients scoring 5 or above were categorized as symptomatic, while those scoring below 5 were considered asymptomatic.
Clinical examination
Relevant clinical parameters were assessed, including respiratory rate (RR), oxygen saturation (SPO2), presence of cyanosis, ability to speak in full sentences, and the use of accessory muscles for respiration.
Computed tomography (CT) scans
The severity index of lung involvement was determined based on CT scan findings.
Laboratory tests
Lymphocyte count and serum ferritin levels were measured to provide insights into patients' immune response and inflammation status.
Clinical outcomes (Fade)
Patient outcomes were tracked and categorized as either "discharged in good condition", "required admission to the Respiratory Care Unit (RCU)," or "deceased.
Ethical considerations
The study was conducted in accordance with ethical guidelines and obtained necessary approvals from relevant institutional review boards. Patient confidentiality and privacy were strictly maintained throughout the study.
Statistical analysis
The collected data was subjected to rigorous statistical analysis to discern trends, correlations, and significant associations. Descriptive statistics were used to summarize the demographic and clinical characteristics of the patient cohort. In addition, inferential statistics were applied to explore relationships between variables and to assess the potential risk factors associated with silent hypoxia in COVID-19 patients.
In conclusion, this multi-center cohort study aimed to comprehensively investigate silent hypoxia in COVID-19 patients by gathering an array of clinical and demographic data. The study design and data collection process adhered to ethical guidelines, ensuring the validity and integrity of the findings.
Results
The analysis of sociodemographic characteristics in relation to hypoxic and non-hypoxic COVID patients, categorized according to hypoxia scores, is presented in Table 1. The mean age for hypoxic and non-hypoxic patients was found to be 52.28 ± 14.93 and 52.79 ± 15.10 years, respectively. Notably, there was no significant difference between the two groups (p > 0.05). Conversely, the mean BMI for hypoxic and non-hypoxic individuals was 30.54 ± 4.52 and 25.43 ± 3.71 kg/m², respectively, displaying a statistically significant difference (p < 0.05). In terms of smoking and gender, no significant differences were observed between the two groups (p > 0.05).
Examination findings of hypoxic and non-hypoxic COVID patients, categorized by hypoxia scores, are presented in Table 2. Among these findings, significant differences were observed in respiratory rate (RR) (p < 0.05) as a significant parameter indicating a high risk, while no significant differences were detected in cyanosis, speech ability, and accessory muscle usage (p > 0.05).
Investigation results for hypoxic and non-hypoxic COVID patients, categorized according to hypoxia scores, are presented in Table 3. No significant differences were observed in SPO2, CT scan findings, lymphocyte count, and ferritin levels between the two groups (p > 0.05).
The clinical outcomes, represented as "fade", in the COVID patients are outlined in Table 4. A substantial proportion of patients exhibited favorable outcomes, with most patients recovering well (78 cases). Importantly, no significant difference was found between hypoxic and non-hypoxic COVID patients with regard to their clinical outcomes (p > 0.05).
Binary logistic regression analysis was performed to explore the relationship between various measured parameters and patient outcomes in terms of being discharged well versus requiring admission to the Respiratory Care Unit (RCU) or experiencing death. The analysis revealed that an increase in BMI was significantly associated with a decrease in hypoxia symptoms, with an odds ratio of 0.636 (95% CI: 0.505-0.801). In contrast, parameters such as lymphocyte count, CT scan findings, ferritin levels, RR, SPO2, smoking habit, and cyanosis showed no significant associations with hypoxia scores (p > 0.05).
Discussion
This study was conducted in Babylon, Iraq, spanning from February to September 2020, focusing on in-hospital COVID-19 patients diagnosed using PCR and non-enhancing chest CT scans. The study cohort consisted of 85 patients, with both hypoxic and non-hypoxic COVID-19 patients exhibiting a mean age of 52.28 ± 14.93 and 52.79±15.10 years, respectively. A notable observation from our study was that hypoxic patients exhibited a higher body mass index (BMI) compared to non-hypoxic patients. This finding aligns with prior research, which has indicated that obesity acts as a risk factor for the severity of COVID-19. Obesity might exacerbate the sensation of dyspnea due to mechanical factors or associated conditions like sleep apnea [22,23]. Our findings emphasize the importance of considering these factors when assessing COVID-19 patients' clinical outcomes.
Regarding examination findings in hypoxic and non-hypoxic COVID-19 patients categorized by hypoxia score, we observed a significant difference between the two groups in terms of respiratory rate (RR). Interestingly, no significant disparity was found in cyanosis, which contrasts with the results reported by William et al. [24]. This discrepancy could potentially be attributed to variations in sample sizes across studies, suggesting the need for further investigations with larger patient cohorts. In terms of clinical outcomes, our study revealed that a majority of the COVID-19 patients (78 cases) experienced favorable outcomes and eventually improved. Importantly, our analysis found no significant difference between hypoxic and non-hypoxic patients in terms of clinical fade. This finding is consistent with research conducted by other scholars [25], strengthening the notion that silent hypoxia may not significantly impact clinical outcomes in COVID-19 patients. It's crucial to acknowledge some limitations of this study. The sample size of 85 patients might impact the generalizability of the results, and further research involving larger cohorts could provide a more comprehensive understanding. In addition, the study's temporal scope might not capture potential changes in patient profiles and disease dynamics over a longer period.
In conclusion, this study offers insights into the clinical characteristics of hypoxic and non-hypoxic COVID-19 patients. Obesity's correlation with hypoxia severity underscores the multifaceted impact of BMI on COVID-19 outcomes. The discrepancies in cyanosis findings warrant further exploration. Ultimately, while silent hypoxia is a notable phenomenon, its influence on clinical fade appears to be limited, emphasizing the need for additional research to unravel its intricacies.
Conclusion
In this study, conducted in Babylon, Iraq, between February and September 2020, we delved into the phenomenon of silent hypoxia in COVID-19 patients. Our findings shed light on various aspects of this condition and its implications: Sociodemographic Insights: Hypoxic and non-hypoxic COVID-19 patients exhibited comparable mean ages, suggesting that age might not be a differentiating factor in the manifestation of silent hypoxia. However, the notable difference in BMI between hypoxic and non-hypoxic patients highlights the potential role of obesity as a risk factor for the severity of COVID-19. This aligns with existing literature that suggests a connection between obesity and disease severity.
- Clinical Assessment Findings: The analysis of clinical assessment findings illuminated a significant difference in respiratory rate (RR) between hypoxic and non-hypoxic patients. This underscores the relevance of RR as a clinical indicator in identifying the presence of silent hypoxia. Additionally, the absence of significant differences in cyanosis, speech ability, and accessory muscle usage implies that these parameters might not serve as reliable predictors of silent hypoxia.
- Clinical Outcomes: The outcomes of COVID-19 patients, as categorized by "fade", indicated that a majority of patients, regardless of their hypoxia status, experienced favorable recoveries. This suggests that the presence of silent hypoxia might not necessarily correlate with adverse clinical outcomes. This finding is consistent with prior research and suggests that clinical management strategies should be comprehensive, taking into account various factors beyond silent hypoxia.
Clinical Outcomes: The outcomes of COVID-19 patients, as categorized by "fade," indicated that a majority of patients, regardless of their hypoxia status,
experienced favourable recoveries. This suggests that the presence of silent hypoxia might not necessarily correlate with adverse clinical outcomes. This finding is consistent with prior research and suggests that clinical management strategies should be comprehensive, taking into account various factors beyond silent hypoxia.
Collectively our study contributes valuable insights into the multifaceted nature of silent hypoxia in COVID-19 patients. Obesity emerges as a potential risk factor for severe disease, emphasizing the importance of patient profile assessment. The discrepancies observed in certain clinical parameters raise intriguing questions that warrant further exploration. While silent hypoxia is a notable aspect of COVID-19, its direct impact on clinical outcomes appears limited. These findings underscore the need for ongoing research to unravel the complexities of silent hypoxia and to enhance our understanding of its implications in patient care and management.
Acknowledgements
The authors would like to thank University of Al-Ameed for the support.
Funding
This is self supported, There is no fund from any institution.
Authors' Contributions
Hawraa M. Kadhim, Ban J. Edan, and Ali Salih Baay: data collection; Abdul Amir H. Kadhum: article formulation; Ahmed Al-Amiery: Analyzed the data.
Conflict of Interest
Authors declared no conflict of interest.
Orcid:
Hawraa M. Kadhim: https://orcid.org/0000-0001-6253-4866
Ban J. Edan: https://orcid.org/0000-0000-2089-9680
Ali Salih Baay: https://orcid.org/0000-0002-7197-0763
Abdul Amir H. Kadhum: https://orcid.org/0000-0003-4074-9123
Ahmed A. Al-Amiery: https://orcid.org/0000-0003-1033-4904
------------------------------------------------------------------------------
How to cite this article: Hawraa M. Kadhim, Ban J. Edan, Ali Salih Baay, Abdul Amir H. Kadhum*, Ahmed A. Al-Amiery, Exploring the clinical implications of silent hypoxia in COVID-19. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(6), 690-700. Link: https://jmpcr.samipubco.com/article_188031.html
------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
