Document Type : Original Research Article
Authors
Department of Orthopedic and Traumatology, Faculty of Medicine, Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia
Abstract
The age-related degenerative joint disease known as osteoarthritis (OA) is typified by a gradual deterioration of cartilage. Once damaged, it is hard for joint cartilage to recover. The secretome is a factor secreted by cells, tissues, or organisms into the extracellular cavity under certain conditions and at a certain time. Studies have shown that injecting secretome into an OA knee can accelerate and stimulate cartilage repair. However, the evidence supporting this claim is limited. This study aims to analyze the effect of secretome injection on the microscopic characteristics of joint cartilage tissue, synovial membrane tissue, and subchondral bone tissue in experimental animals with osteoarthritis. An in vivo experimental study on the Wistar Rat was carried out. To create osteoarthritic conditions, type VII collagenase from Sigma-Aldrich was injected. A total of twenty Wistar rats were divided into two groups: control group and secretome-injected group (cartilage-derived secretome). Each group consisted of 10 rats. After 21 days, the experimental animals were terminated for microscopic analysis of cartilage, synovial, and subchondral bone tissues. The two groups were then statistically compared. It was found that cartilage erosion, subchondral bone erosion, and synovial membrane damage were significantly worse in the control group (p<0.05). It has been discovered that injecting secretome has a positive and protective effect on joints affected by osteoarthritis (OA). The administration of secretome on the osteoarthritic knee was found to be significantly protective, resulting in less damage to cartilage, synovial membrane, and subchondral bone tissue.
Graphical Abstract
Keywords
Main Subjects
Introduction
Elderly people who have osteoarthritis (OA) - a degenerative joint disease associated with ageing - experience pain and incapacity due to osteophyte production, subchondral bone remodeling, and gradual cartilage breakdown [1]. One of the hallmarks of OA is the disturbance of cartilage homeostasis, which is brought on by the breakdown of extracellular matrices such as type II collagen and proteoglycans. According to the previous research, inflammation is a major factor in the development of joint symptoms and the OA advancement [2].
Any substance that cells, tissues, or organisms exude into the extracellular cavity under certain circumstances and at specific times is referred to as a secretome. Genetic material, growth factors, angiogenic factors, hormones, cytokines, extracellular matrix (ECM) proteins and proteases, lipid mediators, and other serum proteins are all found in the secretome [3]. It has been discovered that the secretome's proangiogenic, antiapoptotic, antifibrotic, anti-inflammatory, and immunomodulatory properties can aid in the regeneration of injured joint cartilage caused by early OA [4].
In vivo studies have demonstrated that the application of secretome injection in OA animal models resulted in an improvement of cartilage repair. This process showed an increase in TGF-β, SOX-9, aggrecan, and collagen type II, which are the hallmarks of cartilage repair and regeneration. However, clinical studies utilizing mesenchymal stem cells (MSCs) secretome to treat OA patients are still ongoing and necessary [4].
In this study, we evaluated whether secretome injection was able to influence the microscopic characteristics of cartilage tissue, synovial membrane tissue, and subchondral.
Experimental
Materials and methods
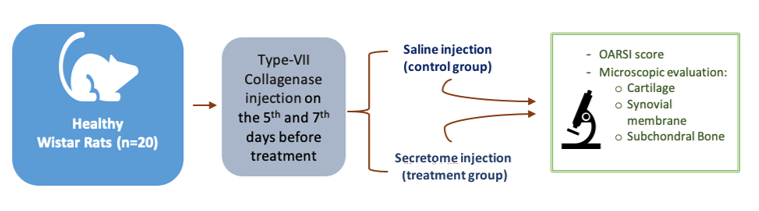
This study used an in-vivo randomized control post-test-only group design. The experimental unit was divided into two different treatment groups, and then evaluated at the same incubation period. The total samples were 20 Wistar rats. The first group was injected with Normal saline (NaCl 0.9%), and the second group was injected with cartilage-derived secretome.
This study was conducted from February to April 2023 in the Animal Laboratory. This research had received approval from institutional animal research ethics.
Healthy Wistar rats weighed 200-300 grams and aged approximately two months without any physical defect were used as samples. The samples were firstly acclimatized to the laboratory environment. Osteoarthritic conditions were made by injecting 3 international units (IU) of type VII collagenase (Sigma-Aldrich), on the seventh and fifth days before the experiment [11]. All intra-articular injections were applied with an injection volume of 6 μL under 2.5% isoflurane anesthesia, using a 50 μL glass syringe (Hamilton Company, Ghiroda, Romania) and 30 G needle (BD).
The stem cells used in this study were previously harvested and stored using the cryopreservation method at the Stem Cell and Tissue Bank of Dr. Soetomo General Academic Hospital. We grew the cell line in a standard culture medium until it reached about 60-70% confluence, and then we washed the cultured cell lines with phosphate-buffered saline (PBS) or serum-free medium (SFM) and incubated them in SFM. After the incubation, we collected the specimen from the conditioned medium. We followed it up with centrifugation and sterile filtration to remove floating cells and cellular debris.
Each sample was randomly allocated to a Control groupwhich was injected with saline, and Secretome group which was injected with secretome. Both groups received three consecutive injections on day 0, day 2, and day 4. On the 21st day, samples were terminated; knee samples were taken, and were analyzed histologically. Synovial membrane, cartilage and subchondral bone will be evaluated based on the Osteoarthritis Research Society International (OARSI) score [5].
Hematoxylin and eosin (HE) staining, cartilage structure, chondrocyte density, and cluster formation are the four factors that make up the OARSI score, which has a total value that can range from 0 to 24. The subchondral bone score, on the other hand, is determined by three factors: the state of the subchondral plate, the formula-based bone volume, and the presence of evident osteophytes. A score of 12 is the maximum that can be attained; a higher score denotes a worse subchondral bone [5].
The compiled data were analyzed statistically using IBM SPSS (Statistical Package for the Social Sciences). In this study, quantitative data was obtained. The normality test was carried out using Shapiro-Wilk. The ANOVA test was used to test whether there were differences between the two groups.
Results
Histopathology
Two blinded observers evaluated each group microscopically, and their summed scores were averaged. The control group showed more erosion and thinner cartilage than the secretome group. The synovial membrane was wider in the control group and the infiltration of inflammatory and fibroblast cells was higher in the secretome group. The control group also had darker color when stained with HE compared to the secretome group (Figure 1).
A 40x magnification of microscopic examination revealed that the structure of the cartilage surface in the control group was more separated. In the control group, chondrocytes and chondroblasts were looser. The synovial membrane in the control group appeared thickened, with abundant infiltration of fibroblasts and neovascularization visible from the endothelial punch (Figure 2).
The OARSI assessment on the synovial membrane, cartilage tissue, and subchondral bone in the control group and secretome group are shown in Tables 1-3. Statistical tests were performed on each OARSI subgroup result (Synovial, cartilage, and subchondral). Normal distribution in the cartilage group of the control (p=0.348) and treatment group (p=0.258) was found. Meanwhile, other groups were not normally distributed. Unpaired t-test or Mann-Whitney test result found a significant difference in treatment and control group on cartilage, synovial, and subchondral (p<0.05).
Discussion
There are biphasic changes in the subchondral bone during the OA development. The initial atrophy phase results in the decrease of subchondral bone volume. Thereafter, the hypertrophic phase begins increasing the bone volume. This explains the mechanism of pain perception in osteoarthritis because cartilage itself is an aneural tissue [6]. In OA, the subchondral bone becomes sclerotic and undergoes pathological microfracture. It is still controversial whether this process is due to the decrease of affect cushioning function of cartilage during its degenerative process or due to the bone changes that proceed and the degeneration of the cartilage. Nevertheless, these changes are in accordance with the degenerative process of the cartilage above them. The border between subchondral and cartilage is calcified cartilage that in OA is proven to cause sensory neurovascular infiltration via osteochondral channel into the area that usually has a poor neurological structure and connects subchondral bone with the cartilage above them and may contribute to the pain in OA.
In this study, we evaluated the cartilage and subchondral bone, and it was seen that in the control group, there was more erosion compared with the secretome group, so the cartilage in the control group is thinner compared with the secretome group. In the control group, matrix destruction was more prominent, also the chondrocyte and the chondroblast were not clearly seen, and were looser compared with the secretome group. In the cartilage, chondroblast cells and chondrocyte cells were denser in secretome group, and this may indicate that dense areas with chondroblast and chondrocyte will undergo better healing.
For the time being, it is still not known the relationship between which factor of the secretome has an antiosteoarthriris effect [7]. According to the research done by Platas et al., it is shown that there is a protective effect from the adipose MSC to the in vitro chondrocyte inflammation model. The in vitro effect is promising and may give more insight into the therapeutic effect of MSC.
In evaluating the synovial membrane done in the control group, the synovial membrane was wider than the secretome group. In the control group, there was more infiltration of inflammatory cells and fibroblastic cells. Compared with the secretome group, the control group is darker in the HE staining. This finding is supported by a study done by Van Buul et al., where there was a tendency to observe an increase in synovial inflammation after the injection of allogenic MSC in rats in the mono-iodoacetate mouse model (MIA). This increase of this synovial inflammation is significant when using xenogeneic human MSC [8], which then leads to the maintenance of MSC immunogenicity. In this aspect, the use of MSC secretion seems safer because the immune complex concentration that may be contained in the extracellular vesicle is lower compared with the cell that affects the weak inflammatory response of the host [9].
Some other literatures support the use of secretome in OA patients. Generally, those studies found that the use of secretome has a positive effect on OA joint, in addition to the low immunogenicity of MSC, and less potential legal issue compared with other cell transplantation cell-based therapy [7,10,12].
Due to the above-mentioned ideas, the biological approach might be an excellent fit for use with humans. In terms of pain relief, morphological changes, and joint protection against OA, the use of extracellular vesicles is just as successful as MSC in the in vivo OA model when secretome is used as a minimally invasive technique [11,12]. Though more individuals are becoming interested, the currently available publications are far from ideal. Although a lot of research points to a broader application of the secretome, little is known about the biological function and underlying role of the secretome. In addition, there is currently a lack of the most appropriate standardization and strategic indication to make use of the prospective biological approach.
Due to limited evaluation period of this study, further research is necessary to assess the long-term effects of secretome treatment on osteoarthritis. In addition, secretomes have the advantage of being freeze-dried, which makes easier and longer storage. This could greatly benefit health centres that do not have access to stem cells. In further studies, the properties of the secretome produced can be analysed by using hypoxia culture techniques in combination with Hydroxyapatite scaffold and Demineralized Bone Matrix [13,14].
Conclusion
This study found that the injection of a secretome derived from cartilage tissue improved the repair of cartilage tissue in an OA animal model, synovial membrane repair, and subchondral bone repair in the same model. The results suggest that further research is needed to re-test the effect of secretome injection on human joint cartilage and to consider secretome as a routine procedure for patients with OA.
Acknowledgements
We would like to express our gratitude to the Department of Orthopedic and Traumatology, Faculty of Medicine, Universitas Airlangga/ Dr. Soetomo General Academic Hospital for supporting this study, and also to Cell and Tissue Bank, Dr. Soetomo General Academic Hospital’s staff for their kind and dedicated assistance during the preparation of the secretome
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Baskoro Kusumo Riswanto: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Experimental studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing.
Dwikora Novembri Utomo: Concepts, Design, Manuscript review, Guarantor.
Lukas Widhiyanto: Concepts, Design, Manuscript review, Guarantor.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Orcid:
Baskoro Kusumo Riswanto: https://orcid.org/0009-0003-2422-9854
Dwikora Novembri Utomo*: https://orcid.org/0000-0002-7832-5695
Lukas Widhiyanto: https://orcid.org/0000-0002-1241-6172
--------------------------------------------------------------------------------
How to cite this article: Baskoro Kusumo Riswanto, Dwikora Novembri Utomo*, Lukas Widhiyanto, Secretome injection effect on microscopic characteristics of cartilage, synovial membrane and subchondral bone in wistar rat knee osteoarthritis model. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(6), 780-786. Link: https://jmpcr.samipubco.com/article_189492.html
--------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
