Document Type : Original Research Article
Authors
KIIT Deemed to be University, Bhubaneswar, Odisha, India
Abstract
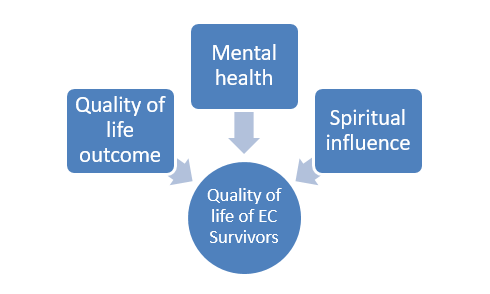
Uterine corpus cancer is a form of gynaecologic malignancy characterized by the excessive and disorganized proliferation of abnormal cells in the uterine lining. Several risk factors, including aging, nutritional imbalances leading to obesity, diabetes, high blood pressure, and nulliparity, have been associated with endometrial cancer. The aim of this study was to assess the awareness level among college-going female students and women residing in rural and urban areas regarding the quality of life experienced by endometrial cancer patients. The data analysis involved the use of a five-point Likert scale and the weighted mean method. The findings revealed that urban respondents exhibited a comparatively higher level of awareness compared to their rural counterparts. It was concluded that the quality of life outcomes, mental disorder, and spiritual influence play a significant role in determining the overall quality of life experienced by survivors of endometrial cancer.
Graphical Abstract
Keywords
Main Subjects
Introduction
Endometrial cancer is expected to affect 66,200 women in the United States by 2023; this figure accounts for 7% of all women with cervical cancer [1]. Among gynaecological malignancies, endometrial cancer is the most common in developing countries [2]. It was previously divided into type 1 (estrogen -dependent, endometrioid) and type 2 (estrogen-independent, non-endometrioid, serous or clear cell) based on hormonal sensitivity [3]. This classification is designed to provide physicians with useful information about epidemic diseases to facilitate timely and accurate diagnosis. Type 1 patients often present with obesity, history of chronic or estrogen therapy, intolerance to progestin, low grade and early presentation, and favourable conditions. On the other hand, patients with insensitivity to type 2 hormones are older, do not have a history of excess estrogen, have decreased sensitivity to progesterone, have high levels, and very painful and have negative effects [4].
EC the most common side effect is vaginal bleeding. It is important to look for abnormalities in women over the age of 35 who have other risk factors such as anovulation, obesity, or postpartum haemorrhage. An endometrial biopsy or D&C may be performed to determine if cancer is present. ACOG now recommends hysteroscopy with D&C to visualize and detect endometrial lesions whenever possible; as this provides the best chance of excluding endometrial cancer. If not performed as part of the D&C, endocardial curettage should be performed to evaluate for uterine infection. For postpartum haemorrhage, ultrasound should first be performed; if the endometrial thickness is less than 4 mm, there is no need to perform a biopsy unless bleeding continues (ACOG) [5]. A negative value D&C should be performed.
The EC diagnosis relates to the degree of evaluation of the glandular components, Grade 1 represents 5% or less non-squamous growth pattern, grade 2 represents 6% to 50% non-squamous growth pattern, grade 3 represents more than 50% non-squamous growth pattern [6].
Endometrial cancer is still unknown, but there are some risks we do know, especially as research continues to better understand this disease. Most endometrial tomurs have been found to have estrogen and/or progesterone receptors on their surface. The interaction between receptors and their hormones leads to endometrial growth, which can be the starting point of cancer. This abnormal growth may continue until it turns into cancer [7].
Long-term exposure to estrogen is associated with most type I endometrial cancers. Estrogen replacement used to control menopausal symptoms increases the risk of endometrial cancer by 2 to 20 times; the longer it is used, the higher the risk. However, the risk of breast cancer can be reduced by simultaneous, continuous or irregular (10 to 15 days per month) administration of progesterone. Effects on unaffected estrogen, such as anovulation (polycystic ovary syndrome), estrogen-producing tumors, and excess androgens converted to estrogen in fatty tissue, also associated with intrauterine Membranous hyperplasia, are associated with an increased risk of breast cancer. Tamoxifen is an estrogen receptor modulator known to act as an estrogen antagonist in breast tissue and an agonist in bone and endometrial tissue. Therefore, tamoxifen use increases the incidence of endometrial cancer by 6-8 times [9-11].
Endometrial cancer in Romania can be significantly affected by obesity. The occurrence of endometrial cancer in obese people can be attributed to the production of endogenous substances resulting from the transformation of fatty tissue. In addition, obese premenopausal women are more likely to experience ovulation. Although there is an independent association between diabetes and endometrial cancer, diabetes has been shown to be associated with an increased risk of endometrial cancer, which may be associated with obesity [11].
Epidemiologically, hypertension is also associated with the risk of endometrial cancer. However, it is unclear whether hypertension is an independent risk factor or whether this association is influenced by medical comorbidities such as diabetes and obesity. Age is another important risk factor for endometrial cancer, and most patients are diagnosed after menopause. Only a small proportion of women (about 15%) are diagnosed before age 50, and an even smaller proportion (about 5%) are diagnosed before age 40. Young women with endometrial cancer are more likely to be obese, nulliparous, and to have well-differentiated endometrioid tumors. Younger women tend to have a lower disease burden and different histology than older women [9].
Factors such as nulliparity, infertility, early menarche, and late menopause are associated with an increased risk of endometrial cancer [12,13].
It is worth noting that the use of combined contraceptives, long-acting medroxyprogesterone acetate, and intrauterine progesterone products may reduce the risk of endometrial cancer. In addition, smoking is associated with reduced endometrial cancer, especially in postmenopausal women [12].
QoL is a matter of great concern for individuals diagnosed with blood cancer and their loved ones. This study delves into various aspects of their well-being, including physical and mental disorder, as well as their socio-economic status. These factors greatly impact their overall quality of life. The findings of the research indicate that younger patients generally experience a better quality of life compared to their older counterparts [14].
Furthermore, family members endure immense suffering if any unfortunate event befalls their dear ones. They are left to navigate through life without the presence of their beloved family member. However, social support, raising awareness, and providing affection can help alleviate these challenges. While we cannot alter destiny, we can strive to provide the best possible care and support to the patient throughout their journey [15]. The study also observed that certain factors such as having children, a mother or sister with a history of ovarian cancer, obesity, late sleeping habits, and undergoing IVF treatment are significantly associated with ovarian cancer. On the other hand, the survey data did not reveal any significant associations between contraceptive pill usage, smoking habits, and a history of diabetes with ovarian cancer [16].
Based on variations in histology and clinical outcomes, endometrial cancers have traditionally been classified into two categories. The majority of endometrial cancers fall under Type I tumors, which are primarily endometrioid adenocarcinomas. These tumors are associated with unopposed estrogen stimulation and are often preceded by endometrial hyperplasia. On the other hand, Type II tumors are predominantly serous carcinomas and are commonly considered independent estrogen [17,18]. They typically arise in atrophic endometrium and originate from intraepithelial carcinoma, a precancerous lesion. Type II tumors generally exhibit lower levels of differentiation and have a less favourable prognosis compared to Type I tumors. Interestingly, despite accounting for only 10% to 20% of cases, Type II tumors contribute to 40% of endometrial cancer-related deaths. The distinct genetic alterations observed in Type I and Type II tumors suggest that these subtypes may have different underlying causes [19,20].
There are several established risk factors that contribute to the development of type I endometrial cancers, primarily involving an imbalance between estrogen and progesterone levels. These factors include obesity and the use of unopposed estrogen therapy. However, the risk of endometrial cancer can be reduced by the use of combined oral contraceptives (OCs), which promote a progesterone-dominant state. Moreover, other risk factors for type I tumors include nulliparity, early onset of menstruation, and late onset of menopause. On the other hand, smoking has been associated with a decreased risk of developing endometrial cancer. In contrast, there is limited knowledge regarding the risk factors for type II tumors, mainly due to the scarcity of cases available for study in epidemiological research [21-23].
Based on the various earlier studies, it was observed that there is lower awareness about the various dynamics associated with EC. The major challenge with the EC that people do not want to discuss the issues with the family members and leads to lower medical attention.
Rationality of the study
In recent times, there has been a noticeable increase in the prevalence of endometrial cancer, which has had a profound impact on the lives of women. This disease not only has financial implications, but also affects the social well-being of patients and their families. After engaging with various NGOs dedicated to this cause, it became evident that there is a pressing need to conduct a study to gauge the level of awareness and perception among female respondents regarding breast cancer. This study would greatly contribute to raising awareness and empowering women to take control of their health.
Scope of the study
The present study is restricted to the rurban areas of Odisha, India. The respondents were basically college going girl students and women of the study areas. The respondents participated not necessarily the EC patients. They include relatives and own family members of different patients. The present study is empirical and survey data being collected with the consent of the respondents without influencing on their opinion and for this no such ethical code was required. The data was collected during the period of 4 months September 2023 to December 2023.
Justification for considering college students
The subject matter primarily revolves around women, thus we decided to incorporate college students who are pursuing their graduation. With the progress in technology, students nowadays possess a profound understanding of various facets of life. Hence, we deemed it appropriate to include this particular group of respondents.
Research design
The current study utilized a cluster and random sampling method. The Likert scale was used for calculation, with the mean serving as the basis for computation. The analysis was conducted with two objectives in mind: assessing the awareness level and evaluating the quality of life. In the awareness data collection, a 4-point scale was employed, while a 5-point scale was used for the quality of life assessment. To collect data on awareness level, a weight of 4 was assigned to fully aware, 3 to aware, 2 to partially aware, and 1 to not aware. For perception collection, the scales ranged from 4 for completely agree to 0 for completely disagree, with 3 for agree, 2 for neutral, and 1 for not agree. The variables considered for the study were identified through an extensive literature review and core group discussions in the study area. The current study included 6 core groups, each consisting of 6 members. For the collection of awareness level data, 12 attributes were considered, while 21 attributes were taken into account for the evaluation of quality of life.
Sample size determination
The sample size for this study was determined to be between 1:4 and 1:10 (Rummel 1970; Schwab 1980). Based on this model, the minimum sample size should be 4 times the item frequency, while the maximum sample size should be 10 times the item frequency. Since we are examining 33 items, 21 for effect of QoL and 12 for awareness of QoL. the minimum and maximum standards should be 132 and 330, respectively. After eliminating biases, we collected 158 observations that met the criteria of falling within the minimum and the maximum values of the sample. According to the guidelines set by Rummel (1970) and Schwab (1980), a sample size of 158 individuals was deemed sufficient for this study.
Refering to Table 1, in the demographic information table, the respondents includes 34 from rural college girls, 46 from urban college girls, 37 were of rural female respondents and rest were urban female respondents out of total of 158 respondents.
Figure 1 represents the awareness level of respondents for various variables by the various respondents participated in the data collection.
Figure 2 repesents the perception of participants for various variables of quality of life and quality of life outcomes by the respondents.
Figure 3 represents the perception of quality of life and mental disorder by the respondents under study. Figure 4 shows the spiritual influence on quality of life opinion by the participants.
Results
Based on Table 2, the mean value for the awareness level was (4+3+2+1)/4 = 2.5. However, the perception mean value of different demographic respondents shows that in case of RCGS and RF the mean values were less than 2.5 which signifies the respondents of this group do not have adequate awareness of endometrial cancer. For the UCGS and UF the mean perception values were more than 2.5 indicates that the participants were much aware of various dynamics associated with the EC.
Similarly, as far as perception of quality of life with reference to Table 3, for the quality of life and quality of life outcomes of EC survivors, the overall mean value was 2 i.e. (4+3+2+1+0)/5= 2. The perception mean value of all the respondents for the various variables among all the groups were more than 2. However, for the VQ2, VQ5, and VQ6 in case of RF the mean perception values were less as compared to other group members. It indicates they do no as strongly believe as by others for these variables considered.
Responding to the variables considered for quality of life and mental disorder the mean value for the same was 2. Majority agree with the attributes considered. However, for the VQ13 and VQ14 the opinion of RCGS and for the VQ14 in case of RF the mean values were less than others. This indicates there was a minor difference opinion with the rest of the groups for these attributes.
In case of quality of life and spiritual influence, most of the groups had positive approach for the attributes but in case of VQ18 and VQ21 for RCGS the mean values were comparatively less than the other group’s responses for these variables. This shows lower acceptability of these attributes among them.
Scope for future study
- Similar studies can be undertaken in other demographic and with different set of respondents.
- The study can be under taken for the women employee of IT sector
- The studies can also be conducted on the women workers working in unorganised sector.
Uniqueness of the present study
The present study is completely different from previous study in the sense, due to different geographic profile, respondents with different back ground and timing. This separates the current study with earlier studies.
Challenges encountered during the study
The major challenge was during data collection, especially interacting women in the rural areas. Due to social customs in the existing rural areas women were reluctant to discuss and participate in the survey. However, we are thankful to local people who facilitated and supported for the completion of data collection.
Conclusion
Endometrial cancer, the most common gynecologic malignancy, is seeing an increase in both the number of cases and death rates. This disease shows significant differences in occurrence and outcomes among different racial and geographic groups. A recent development in the field has resulted in the classification of endometrial cancer into four distinct categories, which are crucial in determining prognosis and guiding treatment decisions. Surgical staging is currently the standard practice, unless the disease has advanced to a later stage, in which case the prognosis and treatment options are accordingly adjusted. The focus of the present study was primarily on the quality of life, indicating the need for increased awareness among rural communities. Despite a lower level of awareness among them, they were still aware of the various challenges to quality of life faced by patients in their area or among their relatives.
Data inclusion and exclusion criteria
The participants in this study consisted of college students, working professionals, and housewives. These individuals were selected based on their willingness to volunteer and participate in data collection activities. Although establishing the criteria for inclusion and exclusion posed challenges, the diligent researchers successfully achieved the desired sample size.
Acknowledgments
The authors express their gratitude to all the participants comprising of rural and urban girl students. Also the opinion provided by the rural and urban female participants for the study. The greatly helped us to complete the paper as per the expected time.
Funding
For conducting the present study, no funding or any financial support received by the authors and the study was undertaken voluntarily with self sponsoring.
Authors' Contributions
All the authors contributed equally for the preparation of the paper starting from conceptual development to data collection, analysis and conclusion.
Conflict of Interest
The present study represents the collaborative efforts of the authors, who affirm that they do not possess any conflicts of interest with regard to any individuals or institutions involved. At present, no financial resources have been procured for the current project.
Orcid:
Deepak Kumar Singh: https://orcid.org/0000-0001-9529-8345
Patnaik, B.Chandra Mohan*: https://orcid.org/0000-0002-5979-0989
Ipseeta Satpathy: https://orcid.org/0000-0002-0155-5548
----------------------------------------------------------------------------------
How to cite this article: Deepak Kumar Singh, Patnaik, B.Chandra Mohan*, Ipseeta Satpathy, Endometrial cancer (EC) and its impact on patients' quality of life (QoL). Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(6), 787-797. Link: https://jmpcr.samipubco.com/article_189493.html
----------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
