Document Type : Original Research Article
Authors
1 Department of Orthopedic and Traumatology, Faculty of Medicine, Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia
2 Department of Anatomic Pathology, Faculty of Medicine, Universitas Airlangga University, Dr. Soetomo General Academic Hospital, Surabaya, 60286, Indonesia
Abstract
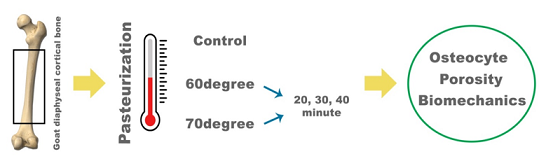
Pasteurization is a relatively easy and effective technique for limb salvage surgery in malignant tumors reconstructed by reusing tissue that has already been infiltrated by the tumor. The pasteurization process affects both tumor cell apoptosis and normal bone cell viability. The study aims to determine the optimal temperature and duration for pasteurization to effectively eliminate tumor cells while preserving the viability and integrity of normal bone. Laboratory experimental studies were conducted to evaluate cell viability, bone porosity, and biomechanical strength of post-pasteurized goat diaphyseal cortical bone. Cortical bones were divided into seven groups with different temperatures and durations. Variations in temperature (60 °C and 70 °C) and pasteurization durations (20, 30, and 40 minutes) were studied. Control groups were used for comparison. A significant difference was found in cell viability and porosity. The highest number of osteocytes was in the control group (80.4%). The osteocytes of the treatment group were the highest at 60 °C and 20 minutes (±77.29%). The lowest was in the group with a temperature of 70 °C and 40 minutes (64.66%). Higher porosity was found in those treated with higher temperatures and longer pasteurization. The biomechanical bending test showed that the force needed to break the bone sample was the lowest at 70°C for 40 minutes. However, the ANOVA statistical test showed no significant difference in all groups (p>0.05). Pasteurization can be used to maximize the eradication of tumor cells yet preserve the normal bone cell and biomechanical strength of the recycled bone autograft.
Graphical Abstract
Keywords
Main Subjects
Introduction
Recycled bone autograft is now the first choice over amputation for the treatment of bone tumors. It is anatomically fit, has no donor site morbidity, no risk of disease transmission, avoidance of immunologic reaction, avoidance complications with prosthesis, and is cheaper than allograft [1,2]. There were several ways to sterilize the autograft, such as autoclave, pasteurization, irradiation, and liquid nitrogen. Pasteurization is one of the easiest ways to do and it is effective in developing countries. It has been said that temperature of 60 °C-70 °C will kill the tumor cells, but it did not destroy the osteoinductive capacity and preserve the biomechanical strength of the bone [3-5]. The duration was still varies between studies. Pasteurization for bone reconstruction after resection of malignant bone tumor was first described by Manabe et al. in 1993. The bone graft was heated in 60 °C saline for 30 minutes and then cooled at room temperature for 10 minutes [6]. Another study by Rong et al. used a temperature of 70 °C for 10 minutes to pasteurize the autogenous bone. Heat treatment at that temperature could eradicate all chondrosarcoma tumor cells. Nakanishi et al. boiled the surgical specimen after resecting all gross tumor tissue at 70±2 °C in 15 minutes [2].
There were a variety of degrees of temperature and pasteurization duration among these studies. As we know, heating does not affect only the tumor cells but also the healthy cells inside the autograft. Each degree of temperature and pasteurization duration definitely have different effects on the bone. A study to measure an effective temperature and duration of pasteurization to kill the tumor cells and also to maintain the viability and integrity of normal bone as an autograft is needed. Therefore, this study aims to analyze the osteocyte, porosity, and biomechanical strength in goat bone as association with human bone autograft in different temperatures and pasteurization durations.
Materials and methods
A laboratory experimental in vitro study using two-year-old goat fresh femur bone. The sample is taken from the diaphyseal region of the femur. The overlying soft tissue was removed. For histopathological study, a tubular bone specimen 1 centimeter in length is cut out. The bone is soaked in a bottle with formalin. For the porosity test, the outer cortical bone is cut 1x1 cm, leaving the medulla. For the biomechanical study, we used a whole femur with a diameter of approximately 20-21 mm and a femur length of approximately 20-22 cm. Every sample is labeled into seven groups: (1) Control (no treatment), (2) P1 (60 °C): P1A (20 minutes), P1B (30 minutes), P1C (40 minutes), (3) P2 (70 °C): P2A (20 minutes), P2B (30 minutes), and P2C (40 minutes).
The bone sample for histopathology evaluation had to be decalcified before paraffin block processing. Decalcification proceeded with formic acid 10%. The solution was changed every 24 hours until the bone felt tender. After the bone felt tender, we continued with the dehydration, clearing, and impregnation process. The dehydration process was done by putting the sample in 70% to 96% alcohol gradually, and then the sample underwent a clearing process by being put in the Xilol. In the impregnation process, the sample was put in the 60 °C solid paraffin for 2 hours. Thereafter, we continued with an embedding process, which is the preparation of base mould and cassette at 60 °C. The base mould that was already filled with the sample was laid on the cold plate for 2-4 minutes, and then the cassette was released from the base mould, and the paraffin block was ready to be sliced in the microtome. The slices were attached to the microscope slide and underwent dewaxing followed by coloring with hematoxylin-eosin.
The method of evaluation of the treatment was described as follows. Histopathological evaluation was proceeded by counting the percentage of osteocyte presence in lacunae. It was taken as an indicator of osteocyte viability after treatment of pasteurization. Evaluation of the bone porosity was initially done by clearing the sample from cells. Bone porosity was evaluated from the enlarged pore size. The pore's diameter was seen on the exocortex of the bone with a scanning electron microscope (SEM) at 1000x magnification. The mean of pore diameter was counted from pores in every four field views (µm). The biomechanical evaluation used the three-point bending test. The whole femur bone was placed in a three-point bending test machine (Shimadzu). The lower support was spanned 10 cm, and the upper indenter was placed in the center between the lower support. The tensile strength of the bone was measured at a speed of 10 mm/s. The mechanical strength of the whole bone will be measured by the maximum load (N) on each sample.
Statistical analysis
The collected data will be described using graphs and tables and analyzed statistically using the SPSS 23 program. For all parameters, a normality test will first be carried out using Shapiro-Wilk. If a normal data distribution is found, the ANOVA test will be continued, whereas if it is not, the Kruskal-Wallis test will continue. In these two statistical tests, if a significant difference is found (p<0.05), a post hoc study is continued to find out which groups specifically differ significantly.
Results
Histopathology
The histopathological examination of bone is displayed in Figure 1, displaying the presence of osteocytes in lacunae. The percentage of osteocyte presence in lacuna is shown in Figure 2. For the treatment group, osteocytes were most seen at 60 °C 20 minutes (P1A) 77.29%. As seen in the bar chart, the percentage of osteocytes declined with the increase in temperature and pasteurization duration.
Statistical analysis using Saphiro-Wilk determined that the data is normally distributed (p>0.05), so the data was analyzed with ANOVA. The ANOVA test showed a significant difference between the treatment groups (p=0.001) (Table 1).
Post hoc analysis showed that pasteurization at 70 °C for 30 and 40 minutes significantly differs from the control group. Osteocyte viability in group 60 °C 20 minutes also significantly differed from 70 °C 30 and 40 minutes. It showed that the percentage of lacunae filled with osteocytes decreases as the temperature rises. The significance of every group is indicated in Table 2.
Bone Porosity
Bone porosity was evaluated from the enlarged pore size. Pore diameter was calculated from the exocortex of the cortical bone. The control group showed zero porosity on the exocortex (Figure 3), while the treatment group showed porosity in various sizes (Figure 4).
The pore diameter is presented in Table 3. The mean pore diameter increased as the temperature and duration of pasteurization increased (Figure 5).
Data distribution analyzed with Shapiro-Wilk was found to be not normally distributed (p<0.05), and then the Kruskal-Wallis test was performed to see the significance. The data was significant (p<0.05) (Figure 6).
Post hoc analysis showed significant differences between the control group and 60 °C 40minutes, 70 °C 20 minutes, 70 °C 30minutes, and 70 °C 40 minutes pasteurization (Table 4). Pore diameter was larger in higher temperatures and had a longer duration.
Biomechanical testing
The result of biomechanical testing of the whole femur was shown to be maximum load, the greatest force to break the bone (Table 5). Table 5 shows that with lower the temperature and the shorter the pasteurization duration, the the strength of the sample is increased. At a temperature of 60 °C and a duration of 20 minutes, it was the most time to match the graft strength before pasteurization (control) at 1495.60. ANOVA test revealed no significant difference in the treated group on the bending test (p>0.05) (Table 6).
Discussion
Histopathology
Reconstruction with pasteurized autograft in tumor cases aims to kill tumor cells by preserving normal bone cells [7]. This study demonstrated microscopic evaluation of cell viability at all temperatures and durations, which were still above 60%. Osteocyte viability at 60 °C for 20 minutes, 30 minutes, and 40 minutes, respectively, were 77.29%, 73.48%, and 70.02%. Temperatures of 70 °C for 20 minutes, 30 minutes, and 40 minutes, respectively, were 75.43%, 65.18%, and 64.66%. Statistical tests found significant differences (p<0.05) for decreased osteocyte viability. The post-hoc test yielded significant differences in the 60 °C group for 20 minutes with 70 °C for 30 and 40 minutes and at 70 °C for 30 and 40 minutes compared to the control. This indicates that a decrease in viability is found at higher temperatures and duration.
As we know, osteocytes play an important role in maintaining bone mass and helping bone remodel during bone healing. These results align with the theory that temperatures below 70 °C can maintain normal cell viability. Research by Yasin (2015) showed that the graft was evaluated microscopically at the end of the 12th week. The pasteurization group had the best outcome in callus formation. This microscopic area contains the most osteocytes and is also present in the bone marrow area of the medullary cavity when compared to autoclaving and irradiation [4]. Sakayama performed a pasteurized autologous bone graft (PABG) mainly at 60 °C for 30 minutes. In this study, 9 out of 10 patients achieved bony union and no local tumor recurrence in the pasteurized bone [5]. Heat treatment at 60 °C for 15 minutes to 10 hours and at 70 °C for 1 hour does not impair bone-inductive activity. This study showed that not only temperature, but also duration of heat treatment were important factors in preserving bone-inductive activity [8].
Another thing to note is the viability of tumor cells, which are also pasteurized. Suwondo et al. compared the viability of tumors treated with pasteurization at 60 °C and 70 °C for 20 minutes, 30 minutes, and 40 minutes. The exposure time of 40 minutes showed a significant difference (p = 0.010) between the 60 °C and 70 °C temperature groups. In this study, no viable tumor cells were found in the 70 °C group with an exposure time of 40 minutes [9].
The temperature and duration that eradicate all the tumor cell yet still maintain the viability of the normal cell is important to this sterilization of the autograft.
Bone Porosity
The effect of pasteurization on bone porosity can be seen from the pore diameter on the cortical bone surface in this study. In the control group, the exocortex surface appeared to be denser and less porous than the treated group. It has been mentioned above that the pore diameter is larger at higher temperatures. Assessing porosity is important because porosity affects the strength of the bone itself. The larger the pore diameter, the more porous the bone will be and, eventually, the more brittle it will be.
Biomechanical
Bone biomechanical strength can be maintained if the collagen from the bones is not damaged. Urist et al. said that temperatures less than 60 °C did not shrink bone collagen, and Vangsness et al. also said that temperatures above 80 °C damaged collagen so that the pasteurization temperature was expected not to affect the biomechanical strength of the bones [10].
The biomechanical test of this study found that the higher the temperature, the smaller the force required to break the diaphysis of the femur. The temperature of 60 °C and 20 minutes was the most time needed to match the strength of the graft before pasteurization (control) at 1495.60 N. However, statistical tests showed that the duration of pasteurization in each temperature group was insignificant for the fracture force in all groups. Singh et al. argued that pasteurization did not significantly reduce strength compared to the autoclave and boiling groups [11]. There was no significant difference in compressive load, compressive stress, and compressive strain when comparing the pasteurization group with the control group [12]. These results align with existing studies that show that pasteurization does not significantly reduce the strength of bone [13].
Conclusion
Pasteurization can be used to maximize the eradication of tumor cells yet preserve the normal bone cell and biomechanical strength of the recycled bone autograft.
Acknowledgements
We would like to express our gratitude to the staff of the Cell and Tissue Bank at Dr. Soetomo General Hospital Surabaya for preparing the bone samples, the Anatomical Pathology Department of RS Dr. Soetomo General Hospital Surabaya, and the Anatomical Pathology Department of Airlangga Medical Faculty staff for processing our histopathological results. Thank you to the Energy and Environmental Laboratory Institut Teknologi Sepuluh Nopember (ITS) staff for processing the porosity evaluation. The last thank you goes to the Materials and Metallurgical Engineering Department staff at ITS for the biomechanical evaluation.
Funding
This study received no external funding agency
Authors' Contributions
Irsa Rahardjo: making the study design, collecting, and analyzing the data, and writing manuscript
Mouli Edward: making the study design, analyzing the data and final approval of manuscript
Ferdiansyah Mahyudin: making the study design, analyzing the data and final approval of manuscript
Heriyawati: processing the histopathological result
Conflict of Interest
The authors declare that they have no conflicts of interest.
Orcid:
Irsa Rahardjo: https://orcid.org/0009-0005-5279-2083
Mouli Edward: https://orcid.org/0000-0002-1667-9770
Ferdiansyah Mahyudin: https://orcid.org/0000-0001-8757-9251
Heriyawati Heriyawati: https://orcid.org/0009-0009-8124-6339
-----------------------------------------------------------------------------
How to cite this article: Irsa Rahardjo, Mouli Edward*, Ferdiansyah Mahyudin, Heriyawati, Pasteurization temperature and duration effect in normal bone osteocyte, porosity and biomechanics: in vitro studies. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(7), 855-864. Link: https://jmpcr.samipubco.com/article_189955.html
-----------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
