Document Type : Original Research Article
Authors
- Mustafa Mudhafar 1, 2
- Hasan Ali Alsailawi 3
- Ismail Zainol 4
- Mohammed Zorah 5
- M.N. Abdulraheem 6
- Murtada M. Hasan 7
- Qais R. Lahhob 8, 9
- Haneen A. A. Oudha 3
- Ali Muhsen Ali 3
1 Department of Medical Physics, Faculty of Medical Applied Sciences, University of Kerbala, 56001, Karbala, Iraq
2 Department of Anesthesia Techniques and Intensive Care, Al-Tuff university college, 56001, Kerbala, Iraq
3 Department of Biochemistry, Faculty of Medicine, University of Kerbala, 56001, Karbala, Iraq
4 Department of Chemistry, Faculty of Science and Mathematics, Universiti Pendidikan Sultan Idris, Proton City, 35900 Tanjung Malim, Perak, Malaysia
5 Department of C. T. E, Imam Al-Kadhum College, Baghdad, Iraq
6 College of Education, University of Anbar, AL Qaim, Iraq
7 Department of Clinical Laboratories , College of Applied Medical Sciences, University of Kerbala, 56001, Karbala, Iraq
8 Department of Clinical Laboratories, College of Applied Medical Sciences, University of Kerbala, 56001, Karbala, Iraq
9 Medical Laboratory Technique, Kut University College, Al-kut, Wasit, Iraq, 52001. College of Health and Medical Technology, Al-Ayen University, Dhi Qar, Iraq
Abstract
Polyalthia rumphii (P. rumphii) is a member of the Annonaceae family with a wide distribution in tropical and subtropical regions. In traditional Chinese medicine, various members of this genus have been employed as medicinal plants to address refractory ailments. The Li ethnic minority, primarily residing in Hainan Island, has traditionally utilised extracts derived from P. rumphii for fever prevention, hypertension management, and the inhibition of cancer cell growth. The current study aimed to extract P. rumphii leaves and identify their chemical components using GS-MS and traditional phytochemical methods. This study evaluated the LPr's biomedical properties using antibacterial and cytotoxicity activities. Three solvents were employed to extract the LPr: hexane, dichloromethane (DCM), and methanol (MeOH). Prepared samples were collected and labelled as HLPr, DLPr, and MLPr, respectively, and their biomedical activities were assessed. The results of phytochemical screening showed the presence of terpenoids and glycosides in all three prepared extracts. At the same time, the alkaloids did not identify as being present in all of them. The results of GC-Mass spectroscopy showed the presence of 22 compounds; the low percentages were Allo-Aromadendrene (0.09%), 2,2-dimethoxyethane (0.27%), and Methyl stearate (0.33%) while Trans-α-bergamotene (11.23%), Benzene, 1-methoxy-4-(4-methyl-4-pentenyl)- (8.97%), 2,5-Pyrrolidinedione, 1-methyl- (6.89%), and 2,6-Dimethylbenzonitrile (5.01%) detected with high percentages. The LPr cytotoxicity was measured against HeLa cells, and the results showed no toxic effect. All of the prepared samples showed a significant impact in inhibiting the bacteria growth. The present study established significant bio-medical properties of LPr and obtained products with nontoxic effect.
Graphical Abstract
Keywords
Main Subjects
Introduction
The Polyalthia genus, which contains over 120 species, is one of the more than 130 genera in the Annonaceae family, which is thought to be the most prominent family [1-3]. Polyalthia rumphii (Blume ex Hensch.) Merr, it is extensively distributed in the tropics and subtropics [4].
The Polyalthia genus is distinguished by its unique effectiveness, which enables it to be employed in the biological and medicinal fields [5]. Previous study has been reported to evaluate their biomedical uses, antioxidant [6], anti-cancer, anti-inflammatory [7], antifungal [8], anti-plasmodial [9,10], anti-DENV2 [11,12] and anti-proliferative [13,14], etc. Previous research was done in several Polyalthia genus parts, such as leaves, roots, stem portions, stems, twigs, and barks, to identify the group's compounds [15]. Different parts of the genus of Polyalthia are traditionally used in medicine to treat a wide range of illnesses, including skin problems, fever, dysmenorrhea, helminthiasis, hypertension, diabetes, and pharyngeal neurosis [16,17].
Many species of Polyalthia is a traditional Chinese medicine that aromatizes dampness, opens the body, and soothes the mind, and is used medicinally. In recent years, a great deal of studies have shown that the chemical constituents of Polyalthia species revealed the presence of several chemical group chemicals, such as terpenoids, tannins, saponins, carbohydrates, flavonoids, alkaloids, etc. Because of their chemical components, and they are considered as a rich in groups of chemical that give them their distinctive efficiency, the Polyalthia species exhibit intriguing biological activities. One member of that genus was picked for this investigation, and its physicochemical and biological activity were examined [18,19].
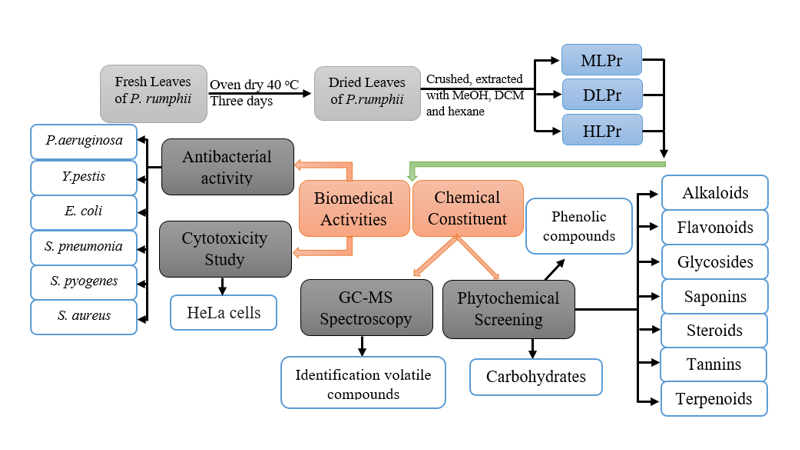
Polyalthia rumphii leaf (LPr) was picked for several reasons, including the fact that no studies have been published to examine their compositions of chemical, cytotoxicity, and antibacterial activity. However, it is possible to obtain comparable phytochemicals from LPr leaves which may have potential antimicrobial activity. In addition, LPr is widely accessible in the area and is inexpensive. Thirdly, because there was little information available on LPr, the current work will serve as a resource for future research. Figure 1 displays the LPs freshness.
In the present study, three solvent systems, i.e. hexane, DCM, and MeOH, were used to extract LPr and its biological activities were evaluated by gauging how well it inhibited six different kinds of bacteria. HeLa cells were also employed for the toxicity study. The results showed that the MeOH extract included carbohydrates, glycosides, terpenoids, and steroids. LPr's biological activity showed that it significantly affects all types (Six) of the bacteria.
Experimental
The Preparation process of LPr
LPr were obtained locally from a proton city, Perak, Malaysia farm. LPr was rinsed in distilled water (D.W) to eliminate all the dust and fungus. Oven dry was used at 40 oC for three days. To obtain LPr powder, the dried LPr was ground carefully and kept for future work. Figure 2 depicts the scheme of the methodology process of the present work.
The extraction method of LPr
Using the hot extraction method, three solvent systems were used to extract LPr, i.e. hexane, DCM, and MeOH.
Briefly, 450 g of LPr were extracted firstly with MeOH to obtain all possible polar compounds, and for the equilibrium polar compound, DCM was used. Finally, hexane was used to remove all potential non-polar compounds, three crude extracts were obtained and labelled as HLPr, DLPr, and MLPr refer to the used solvent hexane, DCM and MeOH, respectively.
The obtained crudes were filtered, dried, and then kept at 4 oC for the next steps.
Chemical characterization of LPr
Prepared samples (HLPr, DLPr, and MLPr) were characterized for their chemical constituent by using Gas chromatography-Mass spectrometry (GC-MS) and classical phytochemical screening technologies.
GC-MS spectrometry analysis of LPr
The chemical constituent of LPr was evaluated using GC-MS (Shimadzu GC-14B) analyser.
The Phytochemical analysis of the LPr
Three extract cured out to evaluate their chemical groups using traditional chemical procedures, as presented in Table 1.
Antibacterial activities of PLr
The prepared samples were treated with six bacteria species to evaluate their ability to inhibition of bacteria growth. Three gram positive include Streptococcus pyogenes (S. pyogenes), Streptococcus pneumonia (S.pneumonia), and Staphylococcus aureus (S. aureus), while the gram negative include Yersinia pestis (Y.pestis), Yersinia pestis (Y.pestis), and Escherichia coli (E. coli).
Agar preparation
2000 mL of DW was used to dissolve 40 g nutrient broth agar, and then an autoclave was used to sterilize the solution at 121 oC for 20 minutes. The solution was then cooled, poured into Petri dishes and left to solidify. After that, the plates were stored at 40 oC and kept for future work.
Prepared samples were cured to evaluate their ability to inhibit bacteria growth using the disc diffusion method. The antibacterial determination for the ready samples was performed using measurement of the inhibition zones. Briefly, 100 µL of the bacteria strains were cultured on the plates by spreading them, and then the prepared samples were put on the 6 mm disc and incubated at 37 oC for 24 hours. Finally, the zones were measured using the rule.
Cytotoxicity study of LPr
The LPr cytotoxicity was determined using Alamar blue assay against HeLa cells. Five concentrations of prepared samples were used, i.e. 50, 100, 150, 300, and 500 mg/ml. The models were cultured with HeLa cells in carbon dioxide (CO2) and incubated for 24 hours at 37 °C.
Results and discussion
The current study was conducted to achieve three objectives. Firstly, extracted of LPr with three different solvents, i.e.
MeOH, DCM, and Hexane, using the hot-extraction method, and three crude extracts were obtained. Secondly, the chemical composition of LPr was determined using screening of the chemical profile and identification of volute compounds using classical screening methods and GC-mass spectroscopy, respectively. The third parameter of the present study aimed to investigate the biomedical properties of the LPr using antibacterial and cytotoxicity studies.
The chemical profile screening evaluated nine groups: Flavonoids, Terpenoids, Tannins, Saponins, Carbohydrates, Steroids, Glycosides, Alkaloids, Phenolic compounds, and Glycosides. The results of the phytochemical profile of MLPr show seven groups out of nine were detected, as demonstrated in Figure 3. Alkaloids. Saponins did not observe to be present.
In the literature, many studies were reported to determine the phytochemical profile of medicinal plants using classical technologies [26,27].
The leaves of the medicinal plants were demonstrated to have various types of chemical groups. The studies of Sadat et al. [28] and Kwansa-Bentum et al.
[29] were reported to extract the leaves of Corchorus olitorius (C. olitorius) and Polyalthia longifolia (P. longifolia) using methanol solvent and evaluated the chemical profile; the results were shown to the presence of saponin, flavonoid, steroid, glycosides, and carbohydrate.
At the same time, tannins were not observed in the leaves of C. olitorius, while P. longifolia showed the presence of saponin and flavonoid, while alkaloids were not detected to be present. The mentioned results are similar to the present study.
Figure 4 demonstrates the phytochemical analysis of DLPr, and the outcome observed the available of steroids, terpenoids, flavonoids, and glycosides. At the same time, tannins, saponins, carbohydrates, alkaloids, and phenolic compounds were not detected to be available.
The leaves of the various medicinal plants were reported to be extracted using DCM to investigate their compositions, and the factional chemical groups were demonstrated to be present [30,31].
DCM was used to extract the leaves of Euodia Redleyi and Adenanthera pavonina. Terpenoids, saponins, and flavonoids were determined to be availably positive, while tannins, phenolic compounds, carbohydrates and saponins were not given positive results [32-34].
The mentioned results were compatible with our obtained results.
Figure 5 illustrates the chemical profile of HLPr which represented to four chemical groups were detected to be presence as a positive test i.e. saponins, tannins, terpenoids, and glycosides while remain five chemical groups were detected to be negative test.
Among the literature, a lot of studies were reported to extract leaves of plants with hexane, and their studies demonstrated various groups to be present, such as flavonoids, steroids, tannins, and saponins [36-38]. The previous results were approximately the same as the current results.
Table 2 provides the overall results for the three extracts, i.e. MLPr, DLPr, and HLPr. The results did not indicate the presence of alkaloids in all sections, while terpenoids and glycosides were observed to be present in all extracts. Flavonoids and steroids were observed in two of three quotes, i.e. MLPr and DLPr.
GC-MS spectroscopy of LPr
The previous part of the current results was indicated by the phytochemical profile of the three extracts, and the extract of MeOH was shown to have seven of nine evaluated chemical groups, more than other solvents. Based on that, MLPr was utilized to assess its chemical volatile compounds using GC-MS.
The results showed the presence of twenty-two compounds. Four of them were indicated with higher percentages, including 2,5-Pyrrolidinedione, 1-methyl- (8), Benzene, 1-methoxy-4-(4-methyl-4-pentenyl)- (9) Trans-α-bergamotene (17), and 2,6-Dimethylbenzonitrile (21) while three compounds were showed lower percentages including 2,2-Dimethoxybutane (1), Methyl stearate (6), and Allo-Aromadendrene (22), as summarized in Table 3.
The twenty-two compounds were drawn using ChemDraw software, as shown in Figure 6. Based on the structures, the obtained compounds were observed with interesting factional groups, such as methyl, hydroxyl, amide, and carboxyl, groups that can give evidence to the activities of the LPr, which make it able to have significant results in biomedical application.
As indicated in Table 4, the chemical-physical properties of all obtained compounds were analyzed using ChemDraw software, including exact mass, molecular weight, mass to charge (m/z), and elemental analysis. Compound number 15 had a higher molecular weight (440.71 g/mol), while compound number 14 had a lower molecular weight (100.16 g/mol). Based on the element analysis, all obtained compounds showed Carbone and hydrogen, which is expected because the extract is considered organic. The other elements obtained are oxygen and nitrogen due to function groups.
Test of cytotoxicity of LPr
The crude extract of LPr was cured to evaluate its cytotoxicity against HeLa cells, as mentioned in the methodology part. Five different concentrations of LPr were used to prepare five samples and treated with the cells. All models showed a no-toxic effect, and the average cell availability was 97.7%, as presentd in Table 5 and Figure 7. Based on the literature studies, the crude extracts of the genus Polyalthia were reported as having a non-toxic effect on the sections of the cells [14]. The current study is constituent with the previous ones.
The evaluation of antibacterial activity of LPr
As mentioned in the methodology part, six species of bacteria were used to estimate the antibacterial activity of LPr. Prepared samples, including MPLS (i), DLPS (ii), and HLPS (iii), were treated with all bacteria species. The ampicillin was used as a positive control, while the solvents (MeOH, DCM, and Hexane) were a negative control; all solvents did not show any inhibition for the growth of the bacteria. The obtained samples showed practical activities against these six species of bacteria. MLPr was indicated to have a higher effect than DLPr and DLPr, where it showed effectiveness, i.e. 11 mm, 8 mm, and 13 mm against S. pyogenes, S.pneumonia and S. aureus, and 8.8 mm, 9.2 mm, and 11 mm against P.aeruginosa, Y.pestis, and E. coli. Figure 8 demonstrates the effect of prepared samples against these bacteria.
According to the results that were obtained from the antibacterial study, the previous study demonstrated the reason that the ability of the medicinal plants to have a significant effect on the bacteria was the functional groups present in the plant; this is due to the diversity in composition of these plants [40-43], based on the chemical analysis of the LPr that mentioned in the previous part, various chemical groups were detected to be present. These groups were responsible for the effectiveness of it against these bacteria.
MLPr was observed to have a higher effect on the bacteria. The reason was observed due to the presence of seven of nine investigated groups, while DPLr showed less impact because only four of nine chemical groups were detected. The current study demonstrated the ability of LPr to be used as an antibacterial agent against both grams of the bacteria (gram-negative and gram-positive).
Conclusion
The current study reported hot extraction of LPr using MeOH, DCM, and hexane. Secondly, its chemical composition was estimated using chemical profile analysis and chemical identification of its compounds. Thirdly, evaluated its biomedical and biological properties. Twenty-two compounds were identified, four of them were detected with higher concentrations, i.e., compound number 8, 9, 17, and 21, while and three compounds showed 1, 6, and 22. As shown in the cytotoxicity analysis, the LPr could be used as an antibacterial agent against six bacteria species, with a non-toxic effect. A current study demonstrated that LPr has various chemical groups with chemical compounds with multiple functional groups. The present study also showed the unique biological properties of the LPr and gave an excellent product without any toxic effects.
Acknowledgements
We are thankful to the editors, reviewers, and participants in this study.
Funding
No funding was received from profit and non-profit organization to do this article.
Authors' Contributions
All authors contributed to the article and approved the submitted version.
Conflict of Interest
This study is the contribution of the authors who do not have any conflict of interest with any other individual or institution.
Orcid:
Mustafa Mudhafar*: https://orcid.org/0000-0002-3785-7396
Hasan Ali Alsailawi: https://orcid.org/0009-0008-5695-7887
Ismail Zainol: https://orcid.org/0000-0001-7491-0419
Mohammed Zorah: https://orcid.org/0000-0003-4255-4338
M.N. Abdulraheem: https://orcid.org/0009-0003-1273-5311
Murtada M. hasan: https://orcid.org/0009-0005-1548-0558
Qais R. Lahhobg: https://orcid.org/0000-0001-5259-8946
Haneen A. A. Oudha: https://orcid.org/0000-0002-6082-2757
Ali Muhsen Ali: https://orcid.org/0009-0001-3142-5699
--------------------------------------------------------------------------------
How to cite this article: Mustafa Mudhafar, Hasan Ali Alsailawi,Ismail Zainol, Mohammed Zorah, M.N. Abdulraheem, Murtada M. hasan , Qais R. Lahhob, Haneen A.A. Oudha, Ali Muhsen Ali, Extraction, identification of chemical constituents, and in vitro evaluation against cells and bacterial pathogens of the leaves of polyalthia rumphii. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(7), 927-943. Link: https://jmpcr.samipubco.com/article_190705.html
--------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
