Document Type : Original Research Article
Authors
1 Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2 Faculty of Health Science, Universitas Muhammadiyah Lamongan, Indonesia
3 Department of Obstetric Gynecology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
4 Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
Abstract
Cigarette smoke contains thousands of compounds that harm active and passive smokers' bodies. These compounds cause oxidative stress in the body. The part of the body that is often affected by exposure to cigarette smoke is female reproduction. Nigella sativa and its constituents have been shown to have antioxidants that can protect the ovaries from damage from cigarette smoke exposure. This study aims to assess the effect of Nigella sativa in ameliorating folliculogenesis due to free radicals from cigarette smoke. Forty-five female Rattus novergicus weighing 150-200 g were divided into five groups. Group 1 Negative Control, group 2 Positive Control (cigarette smoke), group 3 (cigarette smoke+Nigella sativa 300 mg/kg BW/day), group 4 (cigarette smoke+Nigella sativa 600 mg/kg BW/day), and group 5 (cigarette smoke+Nigella sativa 1200 mg/kg BW/day) for 28 days. On the 29th day, the mice were sacrificed and blood was taken from the heart followed by the ovaries. This study showed that Nigella sativa extract increased GnRH levels between the control group and the treatment group (p=0.275), significantly reduced granulosa cell MDA expression (p=0.000), decreased oocyte MDA expression (p=0.000), decreased granulosa cell apoptosis expression (p=0.040), reduced oocyte apoptosis (p=0.005), and increased estrogen expression (p=0.000), increased GDF-9 expression (p=0.0004), and increased the number of follicle graafian (p=0.000). Nigella sativa extract is effective in ameliorating folliculogenesis through granulosa cell MDA, oocyte MDA, GDF-9, estrogen, granulosa cell apoptosis, oocyte apoptosis, and follicular growth due to exposure to cigarette smoke at a dose of 1200 mg/kg BW/day.
Graphical Abstract
Keywords
Main Subjects
Introduction
Folliculogenesis produces a single dominant follicle from a collection of follicles growing in the ovaries. It involves several processes, namely recruitment, development of preantral follicles, selection, and atresia. Folliculogenesis occurs in the ovarian cortex in two phases, namely the preantral phase and the antral phase. The preantral phase is characterised by the growth and differentiation of the oocyte. The antral phase is characterised by a rapid increase in follicle size [1]. If the two phases cannot function according to their functions, folliculogenesis will be disrupted. Folliculogenesis disorders can be caused by PCOS disease, stress, alcohol, and smoking through increased oxidative stress [2,3]. To date, many women who are exposed to passive smoking experience menstrual disorders, implantation failure, risk of miscarriage, low fetal weight, and other reproductive problems [4].
Cigarette smoke containing nicotine, tar, and carbon dioxide can cause oxidative stress. This is due to increased levels of oxidants such as hydroxyl radicals, superoxide anion, and hydrogen peroxide [5]. Hydrogen peroxide reacts with lipid membranes; then lipid membrane peroxidation occurs, causing cell oxidative stress marked by increased MDA [6]. Systemic hydrogen peroxide can affect hypothalamic function, thereby causing GnRH secretion to be inhibited. Inhibited GnRH function can cause LH and FSH stimulation to be inhibited so that ovarian follicles do not grow. LH causes theca cells to release androgen hormones, which are subsequently converted into estrogen in granulosa cells by FSH using the aromatase enzyme [7]. Estrogen plays a vital role as a growth hormone in the reproductive system, is a trigger for ovulation, and functions to maintain oocyte development [8]. If estrogen decreases, it can induce a decrease in Bcl-2 through the estrogen receptor (ESR) signalling pathway. Decreased Bcl 2 will increase Bax gene expression, which causes an increase in caspase 9, caspase 3, and apoptosis [9]. Granulosa cell apoptosis can disrupt folliculogenesis by reducing communication granulosa cells and oocytes, affecting the supply of nutrients and growth factors for oocyte maturation, thereby affecting oocyte quality [10]. Oocytes also play an essential role in coordinating extra- and intrafollicular signals by producing several growth factors such as fibroblast growth factor 8 (FGF8), growth differentiation factor 9 (GDF-9), and bone morphogenetic protein 6 and 15 (BMP6 and -15). For the effects of estrogen on the competent maintenance of cumulus cells during the transition from preantral to antral follicles, GDF-9 and BMP-15 are necessary [11].
The body's defence system, namely endogenous antioxidants, can overcome oxidative stress caused by free radicals triggered by cigarette smoke. However, the body's ability to deal with free radicals is limited, requiring supplements rich in exogenous antioxidants. The natural antioxidant that can be used is Nigella sativa. Thymoquinone is one of Nigella sativa's derived components that can suppress oxidative stress and scavenge radicals [12]. Based on previous research, Nigella sativa extract contains antioxidants such as flavonoids, quercetin, and luteolin [13]. Therefore, this study was intended to assess the protective effect of Nigella sativa on the number of follicles in the ovaries as a result of exposure to cigarette smoke, as well as the previously unresearched impact of cigarette smoke on GnRH levels, granulosa cell MDA expression, oocyte MDA expression, estrogen expression, GDF-9 expression, and oocyte apoptosis.
Experimental
Animals
Forty-five female Rattus norvegicus with a body weight of 150-200 g were obtained from the Rattus Breeding Centre in Malang, Indonesia. After a week of acclimatisation, the mice were kept in a 12-hour light-dark cycle at 23 ± 2 °C and fed a regular diet. All experiments were authorised by the ethics committee of Airlangga University's Faculty of Medicine. Five groups of nine mice were randomly selected from the group of mice. Group 1 Negative Control (no cigarette smoke and no NS), Group 2 Positive Control (CS), Group 3 (CS + NS 300 mg/kgBW/day), Group 4 (CS + NS 600 mg/kgBW/day), and Group 5 (CS + NS 1200 mg/kgBW/day). On the 29th day, the mice were sacrificed, and then surgery was carried out, and intracardial blood samples were taken to check GnRH levels. Blood was taken from the rat's heart by inserting a syringe directly into the heart and slowly sucking it out. The blood tube is placed in the test tube rack and left for approximately 10 minutes. After that, the blood serum was separated using a centrifuge running at 1200 RPM for ten minutes. After that, the blood serum is separated into micro tubes and kept at -20 °C in the freezer [14].
Extraction material
Nigella sativa seeds were obtained from Materia Medica Batu, East Java, Indonesia. Nigella sativa seeds were blended until smooth. 200 mg of Nigella sativa powder was extracted with 1 litre of 96% ethanol. After extraction, the solution is dried with an evaporator to obtain a blackish-brown concentrate, which will be used in the study [15].
GnRH analysis
GnRH analysis was performed following the procedures of the GnRH catalogue. A standard solution containing 0.2 ng/mL was made. The samples and prepared legal solutions were added to the microplate wells in increments of 25 μL each. Subsequently, each well received 200 μL of conjugate enzyme, and the mixture was allowed to sit at room temperature for 60 minutes. Following incubation, 300 μL of washing solution was added to each microplate well to be cleaned thrice. Next, each well received 200 μL of substrate solution. The plates were incubated at room temperature for 15 to 20 minutes. Next, reading is carried out using an ELISA reader [16]. Measurements are carried out with the ELISA kit (FY-ER3820, Wuhan Feiyue Biotechnology).
Immunohistochemical test
For immunohistochemical staining, paraffin was removed by sequentially immersing tissue sections in xylol and ethanol at graded concentrations. The incision was then placed in 3% hydrogen peroxide for 30 minutes and washed for 2 minutes, then treated with 0.025% trypsin for 6 minutes at 37 °C. The incision is treated with primary antibodies (MDA, Estrogen, GDF-9, apoptosis) for 30 minutes and washed with PBS thrice. The incision was treated with secondary antibodies by incubating the incision preparation for 30 minutes. After that, the incision preparation was washed with PBS three times for 2 minutes each and placed in streptavidin/avidin HRP for 30 minutes. The incision preparations were washed with PBS three times for 2 minutes each, rinsed with distilled water, put in Mayer hematoxylin for 6 minutes, washed with running water, and continued with dehydration, clearing, mounting, and observation under a light microscope [17].
Ovarian morphology
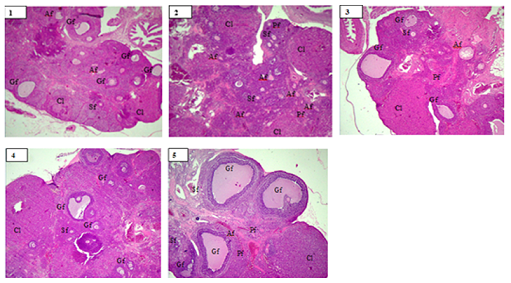
The ovaries were placed in a phosphate-buffered saline dish, dehydrated in acetone at -20 °C for 20 hours, and clarified with metal benzoate and xylene. The samples were penetrated with paraffin. The resulting paraffin blocks were stored at 4 °C until tissue expression was carried out. Samples on paraffin blocks were subjected to serial incisions in 4 sets with a thickness of 3-5 µm each. The first section of each prepared set was stained with hematoxylin-eosin (HE), and then the preantral, antral, and atretic follicles were counted. Observe the structure of the ovaries with 100x magnification using a microscope [18].
Data analysis
Data were tested using the Shapiro-Wilk test to assess whether the data was normally distributed or not because the sample was <50. The information was presented as mean ± standard deviation. A one-way variance analysis (ANOVA) is used to perform a parameter comparison test between the control and treatment groups if the data distribution is normal. The Kruskal-Wallis test compares the parameters between the control and treatment groups if the data are not regularly distributed. Data were analyzed using the EZR version 1.61 program with a significance limit of p<0.05.
Ethical approval
The animal research was reviewed and approved by the experimental design and animal handling procedures as indicated by the animal handling Ethics Committee guidelines at Airlangga University (No.134/EC/KEPK/FKUA/2022).
Results and discussion
Figures 1-7 present the findings of MDA expression, estrogen expression, GDF-9 expression, apoptosis expression, and the follicles morphology. Table 1 presents the mean and standard deviation for each phrase. The results of the Kruskal-Wallis statistical test indicated a significance level of p < 0.05 for data on MDA expression, GDF-9 expression, estrogen expression, and apoptosis expression and p>0.05 for data on GnRH levels. Table 2 lists that Nigella sativa at a dose of 1200 mg/kg BW can improve follicular growth with the most significant mean and ANOVA statistical test p < 0.000.
This study reports the potential effect of Nigella sativa extract against cigarette smoke-induced damage to folliculogenesis in a mice model. Cigarette smoke contains around 4,000 chemicals, most of which have known toxic effects. These chemicals include benzopyrene, nitrosamines, cadmium, nicotine, and aromatic amines, which are polycyclic aromatic hydrocarbons (PAH) [19]. Compounds in cigarette smoke are thought to be able to change hormone function [20]. Nicotine, Tar, and carbon monoxide are all components of cigarette smoke that enter the body and transform into free radicals. An increase in free radicals in the body causes ROS. ROS will cause the GnR protein to change into the protein carbonyl. The protein carbonyl enters the proteasome, thereby causing inhibition of GnRH secretion [21]. The results of this study showed that administration of Nigella sativa extract at a dose of 600 mg/kgBW increased the average GnRH levels. Research results of Bideskan et al. showed that giving Nigella sativa extract to rats with kidney failure could increase the activity of antioxidant enzymes, namely SOD and kidney catalase. Increased SOD and catalase activity can inhibit the occurrence of ROS [22]. The results of other studies also state that Nigella sativa has been proven to provide a protective effect against experimental ischemia-reperfusion injury and has a strong ability to ward off free radicals due to its antioxidant properties [23]. Thymoquinone is an active compound from Nigella sativa seeds with a strong antioxidant effect due to its ability to reduce ROS. Thymoquinone reacts with glutathione (GSH), NADH, and NADPH to form reduced metabolites, such as glutathione-dihydro-TQ, which can scavenge free radicals [24,25]. Oxidative stress conditions, apart from being characterised by a decrease in antioxidant enzymes, are also accompanied by an increase in MDA.
This study revealed the highest mean MDA expression of oocyte and granulosa cells was in the Positive Control group, and the lowest was in the cigarette smoke+NS 1200 mg/kg BW treatment group. Oxidative stress brought on by the presence of cigarette smoke was the cause of the increase in MDA expression in the Positive Control group. This is consistent with a study by Lykkesfeldt et al. which found that smokers' plasma MDA levels were significantly higher than those of non-smokers [26]. Based on this examination, administering Nigella sativa 1200 mg/kgBW can reduce MDA expression in oocyte and granulosa cells. This is in line with the research by Khaldi et al., the administration of Nigella sativa extract caused a decrease in MDA levels in mice exposed to smokeless tobacco [27]. Other research also demonstrated that Nigella sativa can reduce serum MDA levels in mice with experimental head trauma [23]. The decrease in MDA levels was due to the prevention of ROS. This is because Nigella sativa has components such as polyphenols and flavonoids [28].
The findings of Soldin's study indicate that the hormonal system is further impacted by exposure to cigarette smoke. Smoking, both active and passive, can lower a woman's estrogen levels [29,30]. Smoking affects hormones' secretion, synthesis, metabolism, distribution, and secretion. Smoking can drastically reduce aromatase (CYP 19) levels and activity in granulosa cells [31]. The absence of aromatase activity results in antiestrogenic effects. Aromatase deficiency can reduce estrogen concentrations [32]. The average estrogen expression in this study was the lowest in the Positive Control group, and estrogen expression increased further in the Nigella sativa treatment group. Oral administration of Nigella sativa 300 mg/kgBW to mice exposed to cigarette smoke can increase granulosa cell estrogen expression. Results of research Parhizkar et al., where administration of Nigella sativa extract showed estrogenic activity by increasing serum estradiol levels [33]. This uterotrophic activity of Nigella sativa can be attributed to the flavonoid and phenolic compound content of Nigella sativa, which has been shown to have high estrogenic activity [34]. The oocyte-associated granulosa cells become competent to undergo cumulus expansion during the transition from preantral to antral follicles. Estrogen signalling in granulosa cells coordinates with oocyte GDF-9 signalling to promote normal follicle development [11]. GDF-9 can stimulate granulosa cell proliferation. Female mice lacking GDF-9 become infertile because folliculogenesis stops at the primary follicle stage [35]. The results showed that administration of Nigella sativa extract at a dose of 1200 mg/kgBW increased the average expression of GDF-9 in female mice exposed to cigarette smoke. Based on research by Jiao et al., it was found that the administration of quercetin can improve the quality of pig oocytes and recover GDF-9 from aging in vitro due to its antioxidant properties. Quercetin is a component of the flavonoid family [36]. Nigella sativa is rich in antioxidants because it contains flavonoids, quercetin, and luteolin [13]. Apart from that, Nigella sativa and its derivative components, especially thymoquinone, as an antioxidant, have the potential to ward off free radicals and inhibit oxidative stress [12]. The results of the in silico study further showed that the active compounds cholestan-3-ol, 2-methylene-, (3β,5α)-, and cis-13,16-docasadienoic acid could act as candidate activators of GDF-9 and ESR. Therefore, it can increase the production of GDF-9 and ESR [37].
This study showed that the Positive Control group had the highest average expression of oocyte apoptosis and granulosa cell apoptosis. Exposure to cigarette smoke influences increased apoptosis in oocyte and granulosa cells. Administration of Nigella sativa extract in several doses can reduce the expression of oocyte and granulosa cell apoptosis. These results are in accordance with the research of Kamasak et al., that the administration of Nigella sativa after experimental head trauma can reduce oxidative stress products in serum and brain tissue and reduce the level of apoptosis in brain tissue [23]. Another research also states that Nigella sativa extract can weaken apoptosis in yeast Sacchromyces cerevisiae [38].
Assessment of follicle morphology in this study showed that administration of Nigella sativa extract could increase the number of secondary follicles, graafian follicles, and corpus lutheum. These results are by the research by Khani et al., that giving Nigella sativa to PCOS model mice can increase the number of primary, secondary, graphian follicles, and corpus lutheum [39]. Nigella sativa has been reported to have antioxidant properties with its thimoquinone content. Apart from its antioxidant activity, it can protect the ovaries [40]. Estrogen-like activity from Nigella sativa extract in female mice stimulates follicular development and corpus lutheum formation. Follicles are the functional unit of the ovary, and each consists of an oocyte surrounded by somatic cells. To relalize the functions of steroidogenesis and ovulation, the follicle must develop through a series of highly coordinated developmental stages [41]. A woman's reproductive condition can be assessed by the continuity of the ovarian and endometrial cycles. Folliculogenesis is part of the ovarian cycle and is influenced by interactions between oocytes, granulosa cells, and theca cells. This communication provides nutrients and regulatory signals needed for maturation [7].
Conclusion
Administration of Nigella sativa extract can improve oocyte and granulosa cell communication through granulosa cell MDA expression, oocyte MDA expression, GDF-9 expression, estrogen expression, oocyte apoptosis expression, granulosa cell apoptosis, and the number of follicles in female mice exposed to cigarette smoke. Further research is needed to evaluate ovarian changes in theca cells of female mice given Nigella sativa and exposed to cigarette smoke.
Acknowledgements
The authors would like to thank all those who provided useful suggestions and support in this study.
Funding
This study did not receive a specific grant from a funding agency in the public, commercial, or nonprofit sector.
Authors' Contributions
Amirul Amalia: Amirul Amalia contributed to finding concepts, literature search, definition of intellectual content and laboratory experiments. She also contributed to manuscript preparation, editing and finalization of the manuscript.
Hendy Hendarto: Hendy Hendarto contributed to the design methodology and statistical analysis. And he also contributed to the statistical writing and discussion of this article.
Arifa Mustika: Arifa Mustika Focuses on data acquisition and data analysis. He also contributed to drafting and editing the results of this study.
Conflict of Interest
There is no conflict of interest in this research.
Orcid:
Amirul Amalia: https://orcid.org/0000-0001-8697-6474
Hendy Hendarto*: https://orcid.org/0000-0001-5551-2028
Arifa Mustika: https://orcid.org/0000-0001-6461-5782
----------------------------------------------------------------------------
How to cite this article: Amalia, Amirul, Hendarto, Hendy, Mustika, Arifa, Nigella sativa ameliorates folliculogenesis disorders due to exposure to cigarette smoke through gnrh, mda expression, estrogen expression, GDF-9 expression, apoptosis expression, and ovarian follicles. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(7), 997-1009. Link: https://jmpcr.samipubco.com/article_191011.html
----------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company)+ is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
