Document Type : Original Research Article
Authors
- Heroe Soebroto 1, 2
- Ferdiansyah Mahyudin 3
- Gondo Mastutik 4
- Abu Rizal Dwikatmono Johan 2
- Arisvia Sukma Hariftyani 2
- Chabib Fachry Albab 2
1 Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2 Department of Thoracic, Cardiac and Vascular Surgery, Dr. Soetomo General Academic Hospital, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
3 Department of Orthopedic and Traumatology, Dr. Soetomo General Academic Hospital, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
4 Department of Anatomical Pathology, Dr. Soetomo General Academic Hospital, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
Abstract
Bovine pericardium offers regenerative medicine potential in applications like heart tissue repair and implantation materials. To utilize it safely, a decellularization process is crucial to remove cells and antigens. This study aims to compare the characteristics of bovine pericardium decellularized using SDS 0.5% and ASB-16 by evaluating certain parameters. Decellularization was performed with SDS 0.5% and ASB-16. Histological analysis, observation using SEM, immunohistochemistry, and MTT assay were performed. P < 0.05 was considered significant. There were no nuclei in the bovine pericardium group treated with both methods. A picture of collagen was obtained in SDS 0.5%. In the ASB-16, collagen was mild (40%), moderate (40%), and dense (20%). In the group decellularized with ASB-16, GAG was mild (20%), while the other 80% did not show GAG. The mean pore size of the bovine pericardium group decellularized with SDS was 0.5% smaller than that of ASB-16. The differences in type I and type III collagen intensity were not significant. ASB-16 produced significantly more living cells than SDS 0.5%. ASB-16 has better biomechanical characteristics, namely tensile strength (9.0409 N/m2), tensile strain (1.244 m), and young`s modulus (1.56 N/m2). ASB is superior to SDS 0.5% as a decellularization agent in bovine pericardium in terms of pore size, cytotoxicity, tensile strength, tensile strain, and Young's modulus. The bovine pericardium scaffold decellularized with ASB-16 has a larger pore size, less toxic properties, greater strength or tension, greater tensile strength, and less stiff properties than SDS 0.5%.
Graphical Abstract
Keywords
Main Subjects
Introduction
Cardiac surgery for congenital heart disease is an evolving field, with ongoing advancements such as the utilization of pericardial patches. However, segment dissection of the pericardium to create an autologous patch or graft used in the correction of congenital heart disease, makes primary closure impossible in such cases. The pericardium is a thin membrane layer that lines the heart and functions as protection and support for this vital organ [1]. Along with the development of medical science, bovine pericardium (which comes from cows) has become a material that has the potential to be used in various regenerative medicine applications such as heart tissue repair, synthetic pericardium, and providing implantation materials [2]. In an effort to utilize bovine pericardium, it is necessary to carry out a decellularization process to remove cells and antigens that can trigger immunological reactions in the recipient tissue. Decellularization is the process of removing cells and cellular components from biological tissue, leaving only the extracellular matrix remaining [3].
The decellularization process can be carried out using various methods, including the use of surfactants such as Sodium Dodecyl Sulfate (SDS) and ASB-16 (which may be an enzyme or chemical compound). This method has proven successful in the development of decellularized tissue for medical applications [4].
The problems previously discovered were the reduction of glycosaminoglycans and VEGF, as well as damage to the scaffold tissue using SDS as a decellularization agent [5-8]. Meanwhile, ASB-16 decellularization process with ionic detergent is better in maintaining the extracellular matrix because it can retain more collagen, GAG and elastin, while removing 95% of the nucleus and maintaining pore size and porosity [6,9-11].
However, research comparing the differences between the two methods has not yet been conducted. This is important because the decellularization method can influence the resulting extra cellular matrix (ECM) characteristics and impact medical applications and tissue engineering. The aim of this study was to compare the characteristics of bovine pericardium decellularized using SDS 0.5% and ASB-16 by evaluating certain parameters.
Material and methods
Study design, sampling, and population
This research was true experimental type of research using in vitro tests through the posttest-only control group design. The population of this study was bovine pericardium which was randomized, and then divided into three groups. Three groups were established for the study. The first group served as the control, while the second group underwent decellularization using SDS 0.5%. The third group was subjected to treatment with ASB-16.Top of Form The sample size in this study was calculated using the "Resource Equation Approach" formula for exploratory animal research experiments. A sample of 5 was obtained for each group. This research received ethical approval from the Health Research Ethics Committee (KEPK) Soetomo General Hospital Surabaya with certificate number 0306/KEPK/XI/2021.
Inclusion and exclusion criteria
Inclusion criteria include pericardium taken from beef cattle that have been certified as healthy by a veterinarian at the slaughterhouse and scaffolds that have been tested as non-toxic. Samples were excluded from this study if the scaffold experienced defects due to the decellularization process and toxicity tests.
Research material and method
In this study, several variables were employed. The independent variable was the administration of decellularization agents. Dependent variables included nucleus, collagen, GAG, pore size, immunohistochemistry of collagen types I and III, and toxicity tests. Meanwhile, controlled variables encompassed scaffold length, scaffold porosity, scaffold permeability, the method of preparation and the same decellularization method, and the source of pericardium retrieval.
Collecting pericardium specimens
The pericardium collection was carried out at the Pegirian Surabaya Slaughterhouse, which had been standardized following protocols, and the slaughtered animal had been declared healthy by the veterinarian in charge. The pericardium taken was then cleaned by being washed with aquabidest. Once cleaned, the pericardium was stored in 0.9% NaCl transport media at the tissue bank of Dr. Soetomo General Academic Hospital. Later, the pericardium was stored in a freezer at -25 oC.
Decellularization process
The pericardium, which had been cleaned from surrounding tissue and blood contaminants, was then subjected to the decellularization process. The decellularization process is divided into two, namely ASB-16 and SDS 0.5%. In ASB-16, this involves soaking the pericardium in a 3% concentration ASB-16 solution at 4 °C, with the solution changed every 1x12 hours (twice a day) and soaked for 2 days (48 hours). Afterwards, the 3% ASB-16 solution was replaced every 2 x 24 hours, and the process continued for 2 weeks. Subsequently, the pericardium specimen was rinsed using aquabides fluid until it was free from residual decellularized fluid. The pericardium was decellularized using SDS liquid with a concentration of 0.5% at 20-220 °C, 0.5% SDS liquid replaced every 1 x 24 hours for the first 2 days. After that, SDS 0.5% liquid was replaced every 2 x 24 hours, processed for 1 week, 2 weeks, and 4 weeks.
Next, it was placed in the freezer at -80 oC for 1x24 hours and dried (Freeze Dried) with a lyophilizer machine for 2x24 hours. After undergoing the freeze-dried processing, the specimen proceeded with a sterilization process to eliminate contamination by bacterial microorganisms. The sterilization method used was the Gamma-ray radiation method with a dose of 25 Kgray.
Data analysis
The data obtained was tabulated. Afterwards, normality and homogeneity of variance tests were carried out. If the test results show a normal distribution, and then continue with the parametric statistical test using the unpaired T-test with a significance level of 95%. A p-value of less than 0.05 was considered a statistically significant difference.
Results
Differences in Nucleus, Collagen, and Glycosaminoglycans (GAG) in Bovine Pericardium Decellularized with SDS 0.5% Compared to ASB-16
From this test, it was found that there were no nuclei in the bovine pericardium group treated with decellularization using SDS 0.5% and ASB-16. Meanwhile in the control group, 100% of the nuclei showed a mild histological picture. The Kruskal-Wallis test obtained a significance value of 0.001 (<0.05), so it can be concluded that there is a significant difference (Table 1) (Figure 1).
The Mann-Whitney U test showed that there was a significant difference in the histological appearance of the nuclei between the control group with SDS 0.5% (p=0.003) and ASB-16 (p=0.003). Meanwhile, there was no difference between the SDS 0.5% group and ASB-16 (p=1,000) (Table 2).
A picture of collagen was also obtained in the medium category in the bovine pericardium group which was treated with decellularization using SDS 0.5%. In the decellularized group with ASB-16, collagen was mild (40%), moderate (40%), and dense (20%) in the histological picture. Meanwhile in the control group, 100% collagen showed a solid histological picture. The Kruskal-Wallis test obtained a significance value of 0.01 (<0.05), so it can be concluded that there is a significant difference (Table 3, Figure 2).
The Mann-Whitney U Test showed that there was a significant difference in the histological appearance of collagen between the control group with SDS 0.5% (p=0.003) and ASB-16 (p=0.018). Meanwhile, there was no difference between the SDS 0.5% group and ASB-16 (p=0.519) (Table 4).
From this test, it was found that there were no GAG images in the bovine pericardium group treated with decellularization using SDS 0.5%. In the group decellularized with ASB-16, GAG was mild (20%), while the other 80% did not show GAG. Meanwhile in the control group, 100% GAG showed mild histological features. The Kruskal-Wallis test obtained a significance value of 0.004 (<0.05), so it can be concluded that there is a significant difference (Table 5) (Figure 3).
The Mann-Whitney U test showed that there was a significant difference in the histological appearance of GAG between the control group with SDS 0.5% (p=0.003) and ASB-16 (p=0.014). Meanwhile, there was no difference between the SDS 0.5% group and ASB-16 (p=0.317) (Table 6).
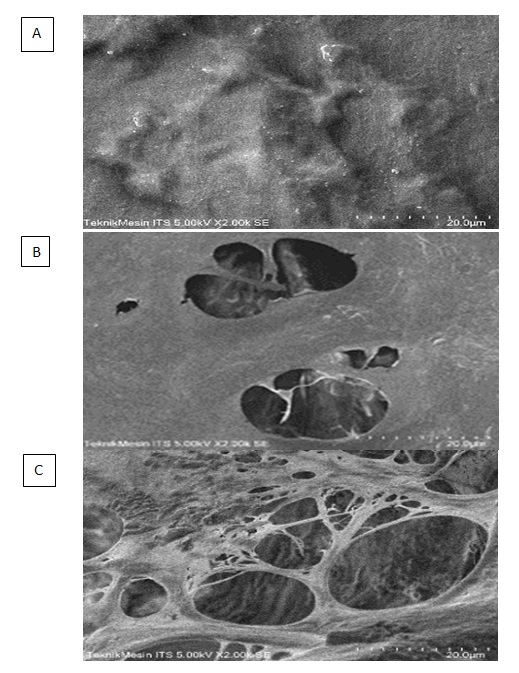
Difference in pore size in bovine pericardium decellularized with SDS 0.5% compared to ASB-16
According to this study, it was found that the mean pore size of the bovine pericardium group decellularized with 0.5% SDS was 7.38 ± 5.03, while the mean of the bovine pericardium group decellularized with ASB-16 was 19.56 ± 11.45. The Mann-Whitney test shows that the)re is a significant difference in pore size between the scaffold groups that were decellularized using ASB-16 and SDS 0.5% (p-value: 0.00002), so it can be concluded that there is an influence of using the decellularization method on pore size in the bovine pericardium (Table 7, Figure 4).
Differences between Type I Collagen and Type III Collagen in Immunohistochemical Examination of Decellularized Bovine Pericardium with SDS 0.5% Compared to ASB-16.
From the Kruskall-Willis test, it was found that there was no significant difference in type I collagen intensity in the immunohistochemical test between the control group and the ASB-16 and SDS 0.5% treatment group (p-value: 0.074) (Table 8).
From the Kruskall-Willis test, it was found that there was no significant difference in type III collagen intensity in the immunohistochemical test between the control group and the ASB-16 and SDS 0.5% treatment group (p-value: 0.086) (Table 9).
Differences in cytotoxicity in decellularized bovine pericardium with sds 0.5% compared to asb-16
From this study, the mean of live cells in the control group was 0.187 ± 0.012, the bovine pericardium decellularized with SDS 0.5% was 0.104 ± 0.002, while the mean of the bovine pericardium decellularized with ASB-16 was 0.236 ± 0.016. Accordingly, it was found that the percentage of living cells on the scaffold decellularized with ASB-16 was 123.15%. This is better than the control group (bovine pericardium without decellularization), which was 82.76%. Meanwhile, in the SDS 0.5% group, the lowest results were obtained, namely 14.15%. The one-way Anova test carried out showed that there was a significant difference in the results of the toxicity test between the control group, scaffolds decellularized using ASB-16, and SDS 05% (p-value < 0.05) (Table 10).
Data were analyzed using post hoc tests by the Howell games method. Accordingly, it was found that the scaffold group decellularized with SDS 0.5% and ASB-16, each had a significant difference from the control. Meanwhile, there was a significant difference between the scaffold groups decellularized with SDS 0.5% and ASB-16 (Table 11).
Biomechanical differences (Tensile strength, Tensile Strain, and Young's Modulus) of bovine pericardium decellularized with SDS 0.5% compared to ASB-16
The control bovine pericardium (without decellularization) was able to withstand a load of 1145.64 N/m2 before tearing, which shows the tensile strength value. The control bovine pericardium had a tensile strain of 0.58 m from its original length. Young's Modulus in control bovine pericardium was 1958.36 N/m2. Bovine pericardium decellularized with SDS 0.5% was able to withstand a load of 3.80 N/m2 before tearing, which shows the tensile strength value. Bovine pericardium decellularized with SDS 0.5% had a tensile strain of 0.45 m from its original length. Young's Modulus in the sample is 8.37 N/m2. Bovine pericardium decellularized with ASB was able to withstand a load of 9.409 N/m2 before tearing, which shows the tensile strength value. Bovine pericardium decellularized with ASB had a tensile strain of 1.244 m from its original length. Young's Modulus of the sample is 7.56 N/ m2.
Discussion
A good scaffold is a scaffold that is well decellularized, so that there are no cells capable of triggering antigenicity. This can be tested by looking at the number of nuclei remaining after the decellularization process. In addition, a good scaffold is expected to still have an extracellular matrix as proven in collagen and glycosaminoglycan (GAG) testing. In this study, researchers attempted to compare control bovine pericardium scaffolds and groups that had been decellularized with ASB-16 and SDS 0.5% in the presence of nucleus, collagen, and GAG.
Differences in the appearance of nucleus, collagen, and GAG in bovine pericardium decellularized with SDS 0.5% compared to ASB-16
Antigenicity remains a major barrier to expanding the use of volatile xenogeneic biomaterials in clinical applications. Foreign body reactions, inflammation, and potential immune rejection can occur due to antigenicity in tissues and organs. The ability of decellularization techniques to solubilize proteins in a nondenaturing manner makes them potentially ideal for antigen removal while avoiding ECM damage [12].
Decellularization is a technique used to reduce antigenicity in a tissue or organ. With the decellularization technique, a scaffold can be formed. This reduction in antigenicity can occur due to the removal of cellular ECM components from tissues or organs. Cell ECM components that are deleted include the nucleus, collagen, and GAG. In this way, it can be concluded that the scaffold can become a compatible tissue or organ due to the organization and remodelling process so that the tissue or organ can function at both a biological and cellular level [13,14].
Moreover, the decellularization method has been proven to reduce calcification caused by the administration of bioprosthetic valves in heart valve surgery so that the duration of valve use is significantly longer [15].
Previous research by Wong et al. [16] and Liu et al. [17] demonstrated that bovine pericardium scaffolds with SDS and ASB decellularization methods could significantly remove ECM cell components, including nucleus, collagen, and GAG when compared with controls. These results are in line with the Kruskal-Wallis and Mann-Whitney U test analysis in this study.
However, there was no significant difference in the scaffolds decellularized using 0.5% SDS with ASB-16.
Differences in pore size in bovine pericardium decellularized with SDS 0.5% compared to ASB-16
Small pore size can cause problems in cell adhesion, proliferation and migration. Therefore, sufficient pore size is very necessary, especially in the application of stem cells in scaffolds. The vacuum method can be added to the bovine pericardium scaffold so that the pore size becomes sufficient [18]. In this study, the pore size of the scaffold decellularized with ASB-16 was significantly larger than that with SDS 0.5%.
Differences in the amount and appearance of type I collagen and type III collagen on immunohistochemical examination of decellularized bovine pericardium with SDS 0.5% compared to ASB-16
Type I and type III collagen have been proven to improve tissue and organ integrity. Decellularization has been proven to increase the intensity of type I and type III collagen in organs so that the biomechanical components become more integrated. This was proven in research on hyaline cartilage scaffolds, chondrogenesis with MSCs and valvular [19-21]. However, in this bovine pericardium scaffold study, the differences in type I and type III collagen intensity were not significant between the control group, SDS 0.5% decellularization, or ASB-16 decellularization.
Differences in cytotoxicity (percentage of live cells) in bovine pericardium decellularized with SDS0.5% compared to ASB-16
Cytotoxicity needs to be tested on transplanted tissues or organs. A study compared the toxicity tests of SDS and ASB-14. According to this study, no difference was found between the lethal dose up to 50% cells (LD50) for ASB and SDS. The toxicity of ASB scaffolds decreased with increasing duration of decellularization [17]. However, in this study, by looking at the number of living cells, significant differences in toxicity were found between the two decellularization methods and the control, but with different results. ASB-16 actually produced significantly more living cells than the others, while SDS 0.5% did the opposite. Furthermore, in an analysis comparing the toxicity of scaffolds with the ASB-16 and SDS 0.5% decellularization methods, ASB-16 was superior in producing a lower level of toxicity with a significantly greater number of living cells. This can be explained by the longer duration of decellularization in this study compared to previous studies.
Biomechanical differences (tensile strength, tensile strain, and Young's modulus) of sidecellularized bovine pericardium with SDS 0.5% compared to ASB-16
Tensile strength shows the amount of force required per area that a material can withstand before tearing. Accordingly, it was found that various decellularization methods showed varying tensile strengths. It was found that there was a decrease in tensile strength in the pericardium scaffold group, whether decellularized with SDS 0.5% or ASB -16. According to a study, decellularized Bovine pericardium has decreased tensile strength [22]. The potential for decellularization assumes that the major cellular immunogenic components have been removed, and that the remaining extracellular matrix (ECM) should retain the required mechanical properties and functional design. However, the decellularization process is likely to alter the mechanical and structural properties of the ECM, potentially affecting long-term durability [23]. Studies show that decellularization procedures show some changes, but may not compromise the strength and mechanical performance of the tissue [24]. The 1 M sodium hydroxide used in PeriGuard decellularization can cause degradation of collagen fibers [25]. The nonlinear response and decreased bending stiffness are most likely related to ECM disruption. Overall, all mechanical measurements were affected by decellularization. This implies that the decellularization process causes mechanical and microstructural defects [23].
Tensile strain shows the change in length of the material per length of the previous material that can be achieved when a force is applied before tearing. In this study, it was found that scaffold samples decellularized with ASB-16 experienced an increase in tensile strain after decellularization, but scaffolds decellularized with SDS 0.5% experienced a decrease. One study found that the overall extensibility represented by area strain under 60 N/m increased from 68.85% for native aortic valves to 139.95%, 137.51%, and 177.69% for SDS, Trypsin, and Triton X-100, after decellularization [23].
Young's Modulus shows the stress per strain that a material can achieve. The smaller the Young's Modulus value, the more elastic a material is. In this study, it was found that the elastic modulus of samples decellularized with either SDS 0.5% or ASB-16 decreased compared to the native samples. The decellularization process reduces the elastic modulus. In one study, it was stated that bovine pericardium that was decellularized had a reduced Young's Modulus compared to that that was not [22].
Another study found that the flexibility of the decellularized aortic valve showed an overall loss of stiffness, and also produced a nonlinear moment-curvature relationship compared to the linear response of the original aortic valve [23].
Conclusion
This study found that ASB-16 was superior to SDS 0.5% as a decellularization agent in bovine pericardium in terms of pore size, cytotoxicity, tensile strength, tensile strain, and Young's modulus. Meanwhile, the ASB-16 ability compared to SDS 0.5% in cleaning the nucleus and maintaining collagen and GAG is just as good. The ability to maintain type I and type III collagen in immunohistochemical tests was also the same between ASB-16 and SDS 0.5%.
Acknowledgements
We acknowledge the department of thoracic and cardiovascular surgery for providing space and staff for discussions so that this paper could be completed.
Funding
None.
Authors' Contributions
Heroe Soebroto, Ferdiansyah Mahyudin, Gondo Mastutik: conception and design of the research, acquisition of data, analysis and interpretation of the data, writing of the manuscript, and critical revision. Abu Rizal Dwikatmono Johan, Arisvia Sukma Hariftyani, Chabib Fachry Albab: writing of the manuscript and critical revision.
Conflict of Interest
Nothing to declare.
Orcid:
Heroe Soebroto: https://orcid.org/0000-0001-7422-2624
Ferdiansyah Mahyudin*: https://orcid.org/0000-0001-8757-9251
Gondo Mastutik: https://orcid.org/0000-0002-1681-0222
Abu Rizal Dwikatmono Johan: https://orcid.org/0000-0003-0016-4841
Arisvia Sukma Hariftyani: https://orcid.org/0000-0001-5872-7104
Chabib Fachry Albab: https://orcid.org/0009-0009-5959-4900
-----------------------------------------------------------------------------------
How to cite this article: Heroe Soebroto, Ferdiansyah Mahyudin, Gondo Mastutik, Abu Rizal Dwikatmono Johan, Arisvia Sukma Hariftyani, Chabib Fachry Albab, ASB-16 was superior to SDS 0.5% as a decellularization agent: an in vitro study using bovine pericardium. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(8), 1028-1041. Link: https://jmpcr.samipubco.com/article_191787.html
-----------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
