Authors
- Nur Rochmah 1
- Ike Wahyu Triastuti 1
- Wika Yuli Deakandi 2
- Roedi Irawan 1
- Muhammad Faizi 1
- Anang Endaryanto 1
1 Department of Child Health, Faculty of Medicine Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia
2 Faculty of Medicine, Universitas Islam Malang, Malang, East Java, Indonesia
Abstract
Type 1 diabetes mellitus (T1DM) is an autoimmune disease that often affects children. This condition is caused by a failure in peripheral tolerogenic mechanisms. The role of transforming growth factor-beta 1 (TGF-β) in this mechanism is still controversial. This study evaluated the association between serum TGF-β levels and T1DM in children. This study may serve as a foundation for exploring the potential benefits of TGF-β knowledge, such as the discovery of novel therapeutics or the prevention of complications associated with T1DM. A case-control study was conducted with 26 children with T1DM and 26 without T1DM (as control) at the General and Endocrinology Pediatric outpatient clinic and pediatric ward of Dr Soetomo Surabaya Hospital from October 2020 to March 2021. Differences in serum TGF-β levels were determined using the Mann-Whitney U test. The mean age of onset was 7.23±4.11 years, and the time duration of diagnosis was 6.35±3.45 years. TGF-β was lower in T1DM than in the control group (1.36, 95% CI 0.44–3.42 ng/mL vs. 3.54, 95% CI 2.01–4.00 ng/mL; p
Graphical Abstract
Keywords
Introduction
Type 1 diabetes mellitus (T1DM) is the most common autoimmune disease that affects children [1]. The T1DM prevalence varies by race and ethnicity. The global prevalence is around 1.1 million, and "Indonesia had around 1,249 children diagnosed with T1DM from 2017 to 2019 [1,2]. According to the International Diabetes Federation, the prevalence of diabetes mellitus is predicted to increase from 9.3% or 463 million in 2019 to 10.3% or 578 million in 2030 [1]. T1DM occurs due to damage to pancreatic β cells via immunological reactions [3]. The course of autoimmune T1DM begins with genetic susceptibility accompanied by environmental exposure, which causes immune system dysregulation, with the target organ the pancreatic islet. This dysregulation consists of a failure in tolerogenic processes, the imbalance of pro-inflammatory and anti-inflammatory cytokines, and the formation of autoantibodies [4,5].
Transforming growth factor-beta (TGF-β) is a pleiotropic cytokine that plays an important role in controlling cell proliferation and altering immune cell activities [6,7]. The TGF-β plays essential roles, including in the proliferation, apoptosis, differentiation, and function of islet β cells [7]. In T1DM, TGF-β acts as a mediator of Treg activity causing T-cell suppression, leading to immune tolerance and decreased production of pro-inflammatory cytokine. On the other hand, TGF-β promotes the synthesis of extracellular matrix (ECM), which contributes to extracellular remodeling in peripheral organs and induces micro- and macro-vascular chronic complications [8]. Additional biomarkers are necessary to evaluate β-cell destruction and T1DM progression. Because of this contradictory effect, the TGF-β biomarker is interesting for early detection and the emergence of chronic complications [9,10]. Because the TGF-β signalling system is linked to diabetes development, insulin resistance, and autoimmune in type 1 and 2 diabetes, as well as the complications, therefore drugs targeting TGF-β signaling may be potential alternatives to conventional diabetic treatments [11].
The study of the TGF-β biomarker in T1DM is still limited. Therefore, we aimed to investigate the association between TGF-β1 and T1DM in the population of Indonesian children with T1DM. This study may serve as a foundation for exploring the potential benefits of TGF-β knowledge, such as the discovery of novel therapeutics or the prevention of complications associated with T1DM.
Experimental
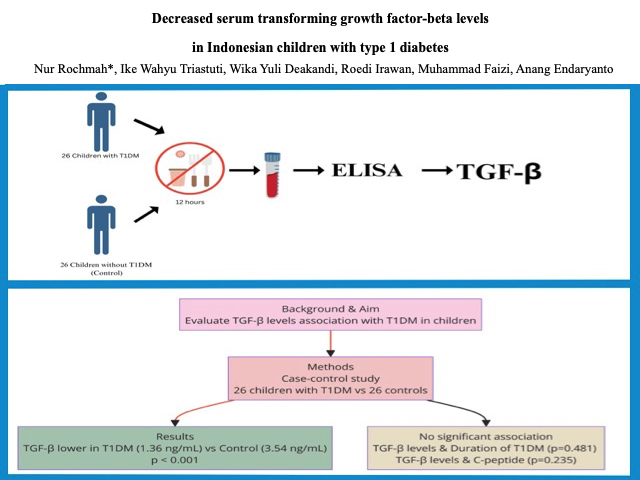
This was a case-control study comparing serum TGF-β levels between children with T1DM and without T1DM (control). This research was conducted at the pediatric endocrine clinic and pediatric inpatient installation in Dr. Soetomo Hospital Surabaya from October 2020 to March 2021. It included 26 children with T1DM and 26 without T1DM. The inclusion criteria for the T1DM group were children diagnosed with T1DM determined using the ISPAD Clinical Practice Consensus Guidelines criteria 2014, aged 2-18 years, and consented to join this study. Meanwhile, children without T1DM history or another autoimmune disease who visited the pediatric outpatient clinic (OPC) and were willing to participate in this study were recruited as a control group. Children with T1DM hospitalized in the pediatric critical care unit were excluded. This study was approved by the Clinical Research Unit at the Dr. Soetomo Hospital in Surabaya, Indonesia, with the ethical number 0154/KEPK/II/2021.
Serum TGF-β levels were measured using the enzyme-linked immunosorbent assay (ELISA) method with LEGEND MAXTM total reagent Human Latent TGF-β ELISA Kit. A 100-µL blood sample was collected from peripheral serum in participants who had fasted for at least 12 hours, and collected in a BD Vacutainer blood sampling tube. Serum TGF-β results were measured in ng/mL units. The odd ratio (OR) and 95% confidence interval (CI) were used to analyze the serum TGF-β between T1DM and control. All statistical analyses were performed using SPSS version 20.0. A significant difference is indicated by a p-value of <0.05.
Results and discussion
This study recruited 52 participants divided into pediatric patients with T1DM (26 children) and without T1DM (26 children) as controls. The percentage of girls was 46.2% in both groups and the percentage of the boys was 53.8%. The mean age of children with T1DM was 13.40±3.53 years, with the highest incidence in children above 12 years old (76.9%). The mean weights were 37.3±13.89 and 30.63±10.64 for the T1DM and control group, respectively. The mean height was 138.62±18.49 for the T1DM group, slightly taller than the control group which was 131.12±13.17. The mean BMI was 18.71±417 for the T1DM group and 17.28±3.34 for the control group. The mean onset of T1DM was at the age of 7.23±4.11 years, and the mean duration of condition for T1DM was 6.35±3.45 years (Table 1).
The serum TGF-β levels in T1DM were significantly lower than those in the control group [1.36 (0.44-3.42) vs. 3.54 (2.01-4.00)], respectively (p<0.001) (Table 2). This finding is similar to the result of Roohi et al., who showed that serum TGF-β levels were statistically lower in T1DM than in healthy controls (p=0.001) [12]. A meta-analysis study by Qiao et al. showed the same result (SMD, -1.97; 95% CI -3.79 to -0.15; p=0.030) [12]. The correlation between serum TGF-β levels and disease duration was not significant (p=0.481). Furthermore, the correlation between serum TGF-β levels and c-peptide was also not significant (p=0.235).
The conditional duration in this study did not significantly correlate with the level of serum TGF-β (p=0.481). In contrast, a study by Azar et al. described that serum TGF-β levels decreased significantly in T1DM, especially in children with a duration of condition of more than two years [6]. Furthermore, other studies by Zorena et al. stated that children with more than 15 years of disease increased the risk of microangiopathy complications by 25% [14]. The TGF-β downregulation was previously stated in the PMBC of children with T1DM, which suggests that individuals with long-term T1DM have lowered immunity [15]. In the early stage of T1DM, the decrease of serum TGF-β levels is masked by hyperglycemia. Subsequently, after two years of the disease, the TGF-β suppression was more severe, which resulted in less evident stimulation by hyperglycemia [6]. The low level of TGF-β may lead to immune alteration and insufficiency of immunosuppression, contributing to the propagation and maintenance of the disease [6].
In this study, the serum TGF-β levels did not correlate with c-peptide significantly. This result is similar to a study from Szypowska et al. that explained that there is no correlation between TGF-β levels and c-peptide in T1DM children [16].
TGF-β is a multifunctional cytokine that influences differentiation, development, cell growth, and immune system modulation [17]. The role of TGF-β due to T1DM pathophysiology is to induce a tolerogenic T-cell response to prevent the disease. Multiple animal pancreas-specific models demonstrated that inhibition of diabetes development can occur caused by the overexpression of active TGF-β [17].
The dysregulation of the TGF-β pathway is associated with the progression of complications, delayed wound healing, and glomerular sclerosis [11]. Interestingly, a study by Zorena et al. mentioned that the serum TGF-β level was significantly higher in T1DM patients with nonproliferative diabetic retinopathy (NPDR). This finding demonstrates that TGF-β might be a further parameter in predicting the prevalence of complications in young children with T1DM such as NPDR [14]. Moreover, another study showed that TGF-β might be helpful as a biomarker of the progression NPDR in people with diabetes [19].
The mean onset of diagnosed T1DM in this study was 7.23±4.11 years old. During the period 2000 to 2017, the highest prevalence of T1DM was in 5-9-year-olds. Environmental factors may play an important role [20].
This study also has limitations, such as the small sample size, although it was conducted in a referral hospital in East Indonesia. According to several studies, prevalence of T1DM in Asia is lower than that in Caucasians [21-27]. Moreover, further study about T1DM complications and their correlation with serum TGF levels is highly recommended.
Conclusion
The serum TGF-β levels were significantly lower in the T1DM group than in the control group. The low TGF-β levels in children with T1DM may indicate the immunocompromised state.
Acknowledgments
The authors would like to thank to all the study participants and the endocrine team of Dr. Soetomo General Hospital, Surabaya, Indonesia, for their support.
Funding
This research is self-funded.
Authors' Contributions
NR, WYD, and IKT conceptualized the methodology and software used, analyzed data, interpreted results, and wrote the initial draft of the manuscript. RI and NR analyzed data, interpreted results, and assisted in drafting the manuscript. AE and MF critically reviewed and edited the manuscript. They also guided the manuscript writing. IKT and RI collected the data, prepared the figures and tables, and analyzed the data. All authors agreed and gave final approval to the submitted manuscript.
Conflict of Interest
The author declares no conflicts of interest in this study.
Orcid:
Nur Rochmah: https://orcid.org/0000-0002-9626-9615
Ike Wahyu Triastuti: https://orcid.org/0009-0001-8986-5208
Wika Yuli Deakandi: https://orcid.org/0000-0002-5656-8725
Roedi Irawan: https://orcid.org/0000-0001-5999-4773
Muhammad Faizi: https://orcid.org/0000-0002-7009-4896
Anang Endaryanto: https://orcid.org/0000-0002-7988-2274
-----------------------------------------------------------------------------
How to cite this article: Nur Rochmah, Ike Wahyu Triastuti, Wika Yuli Deakandi, Roedi Irawan, Muhammad Faizi, Anang Endaryanto. Decreased serum transforming growth factor-beta levels in Indonesian children with type 1 diabetes. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(9), 1478-1484. Link: https://jmpcr.samipubco.com/article_194954.html
-----------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
