Document Type : Review Article
Authors
- Heba Fawzy 1
- Nermin Saad 2
- Khaled Ahmed Elbanna 2
- Huda E. M. Said 3
- Rania Yehia Helal 4
- Hend Sameh 1
1 Medical Biochemistry Department, Faculty of Medicine, Zagazig University, Egypt
2 Internal Medicine Department, Faculty of Medicine, Zagazig University, Egypt
3 Clinical Pathology Department, Faculty of Medicine, Zagazig University, Egypt
4 Neurology Department, Faculty of Medicine, Zagazig University, Egypt
Abstract
Atherosclerotic vascular disorders and its sequalae, involving myocardial infarction and stroke, are major reasons of human diseases and death globally. Moreover, carotid intima media thickness (CIMT) measurement is used to evaluate subclinical carotid atherosclerosis. Therefore, indicators are necessary for prediction of subclinical atherosclerosis. Earlier research have exposed that miRNA-29a could show an imperative role in atherosclerosis and levels of miR-29a were positively related with CIMT. Although no information concerning the levels of miR-29a or miRNA 146a and their correlation with CIMT is available, the objective of our study is to investigate the role of plasma miR-29a and miR-146a values as potential biomarkers of carotid atherosclerosis and to correlate them with CIMT. The values of miR 29a and miR 146a were measured in plasma from 50 healthy volunteers (group I) and 50 patients with CIMT greater than 1.2 (group II) by quantitative real time –PCR (qRT-PCR). CIMT was measured by carotid Doppler to detect atherosclerosis in both groups. Significant upregulation of miR 29a and miR 146a levels in group II than group I. A positive correlation was demonstrated between both miR 29a and miR 146a with CIMT and CRP, respectively. ROC curves of combined miR 29a and miR 146a to detect CIMT ˃ 1.2 showed sensitivity and specificity of 74.4% and 100% particularly. The combination of both miR 29a and miR 146a could act as promising biomarkers of carotid atherosclerosis in risky people, hence accelerating the diagnosis for proper management of atherosclerotic progression.
Graphical Abstract
Keywords
Main Subjects
Introduction
Atherosclerotic vascular disorders and its clinical sequalae, involving myocardial infarction and stroke, stand to be the major reasons of human morbidity and mortality globally sorts [1]. Simultaneously, large vessel diseases, which include ischemic heart disease (IHD), carotid artery disease and peripheral vascular disease, implicate harm to large vessels and cause significant reactions and indexes of atherosclerotic vascular diseases, for instance carotid artery arteriosclerosis [2]. It is generally approved that amplified carotid intima media thickness (CIMT) is an atherosclerosis indicator. Besides, this is frequently regarded the utmost apparent and vital indicator of subclinical atherosclerosis [3,4].
CIMT was revealed to expect cardiovascular hazard in numerous large cohort research and can help for recommending asymptomatic adults with medium hazard of cardiovascular diseases for atherosclerosis hazard evaluation [5,6]. Atherosclerosis progress is complex, but one vital theory is that atherosclerosis is originated by the infiltration of vessels by numerous cells of inflammation and/or its response [7].
Lately, it has been shown that microRNAs (miRNAs) show significant roles in progress of atherosclerosis [8]. MiRNAs are a type of non- expressing, single-stranded RNA fragments which are tangled in definite post- transcriptional control of gene expresion [9]. MiRNAs have arisen as main performers in a varied range of biologic courses, and variation in their level is related with numerous human diseases [9,10]. Researches have displayed that microRNAs adjust numerous cytologic and molecular procedures linked with atherosclerosis progress, starting from hazard factors, to plaque formation and development, reaching to plaque break [11].
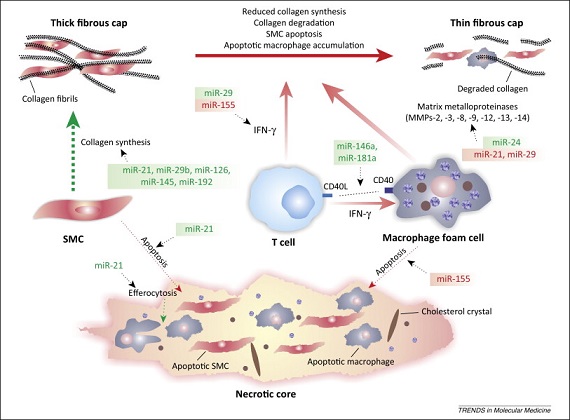
Prominently, earlier research have exposed that miR-29a could have an imperative role in atherosclerosis progress [12]. Moreover, it has been proposed that miR-29a can have vital effects in atherosclerosis progress. Huang et al. (2020) demonstrated that the values of miRNA-146a in the plasma of the CAS cases were greater than normal group. Besides, no information concerning the levels of miR-29a, or miRNA 146a and their correlation with CIMT have been investigated [13]. The aim of the present study is to study the role of Plasma miR 29a and miR 146a levels as potential biomarkers of carotid atherosclerosis and correlate them with CIMT and CRP.
Subjects and methods
Research subjects
Between July 2023 and December 2023, this case-control study was performed at Zagazig Faculty of Medicine Medical Biochemistry and Clinical Pathology Departments, Egypt. Fifty (50) carotid atherosclerotic CAD patients were gathered for the research at Internal Medicine Department .They possessed at least one hazard factor for Atherosclerosis e.g., smoking, DM, or family history of hyperlipidemia. Carotid intimal media thickness of common carotid artery (CIMT) was calculated via ultrasound Toshiba (Aplio xv model) high resolution machine, pulsed Doppler B mode with frequency broad band 614 MHZ .The case group had CIMT ˃ 1.2. Fifty healthy volunteers with matching age and sex shared as a control group who had CIMT˂1.2. A printed consent was obtained from each candidate before the study. Patients with heart failure, previous stroke, transient ischemic attacks in the previous 3 months were excluded from the study. The research was executed in agreement with Declaration of Helsnki .The study was agreed by the IRB Committee with approval number (ZU-IRB # 10728 /4-6-2023). The following was implemented to all participants: full history, clinical examination, and lipid profile.
Method
Collection of venous samples
5 mL of blood samples were obtained and divided in 2 tubes (1 mL in plain tube for serum collection for CRP measuring) and 4 mL on EDTA for real-time PCR of plasma miR-29a and miR-146a values. MiRNA extraction was via utilizing miRNeasy kits from Qiagen. Every phase was implemented following the directions of the kit.
Production of cDNA
Reverse transcription was implemented by mi Script IIRT kit Qiagen, The cDNA were transferred to a -80 ºC freezer.
Amplification for miRNA values
The amplification was applied in a twenty µL combination containing 5µL of the cDNA, one hundred pmol/mL of every primer miRNA- 29a (UAGCACCAUCUGAAAUCGGUUA), miRNA 146a (CCTGAGAAGTGAATTCCATGGG) or RNU6 )CTCGCTTCGGCAGCACAT), ten µL SYBER Green PCR Master Mix and four µL distilled H2O. The amplification was applied by Real time Cycler (Strata gene Mx3005P) as stated in the following strategy; primary start step 95 ºC for fifteen minutes followed by 40 rounds of 95 ºC for fifteen seconds, 55 ºC for 30 seconds and lastly 70 ºC for thirty seconds. The scale of variation of the miRNA value noticed in patients when compared to controls was assessed by the 2-ΔΔCt method.
CRP measurement
It was measured using CRP-human ELISA kit from Thermofisher.
Lipid profile
It was measured using Cholesterol Assay Kit – HDL, TGs, and LDL (catalogue N:1001091) from SPINREACT,S.A,Spain.
Statistical analysis
Data analysis was done using SPSS version 20. We assessed the tests of significance between the groups by ANOVA and Chi-square tests (P- value is significant when ˂ 0.05). Also, we performed ROC Curve to recognize cutoff value to recognize patients with CIMT ˃ 1.2.
Results
Table 1 shows demographic data among the study population. Total of 100 individuals were subdivided into 2 groups, Control group (50 individuals, 48.94 ± 4.44 years, 44% males vs. 56% females ) and patient group (50 patients, 49.04 ± 4.11, 46% males vs. 54% females) with no statistical significant difference regarding age and gender. Regarding risk factors among the study population, there was a significant variance between the groups under study as regard as smoking, hypertension, diabetes mellitus and family history. Regarding smoking, 34% of patients are smokers. Regarding HTN, 72% of the patient group was hypertensive. Concerning diabetes mellitus, 50% of the patients were diabetics while family history, 22% of the patients had positive family history of hyperlipidemia.
Table 2 presents lipid profile and fasting blood sugar among the study population. Fasting blood sugar in Control group mean ± SD was 93.96 ± 13.92 while in patient group the Fasting blood sugar mean ± SD was 118.58 ± 14.01 with highly statistical considerable variance (p= <.001 ) between the two groups. Total cholesterol in Control group mean ± SD was 151.73 ± 9.11 while in patient group, the total cholesterol mean ± SD was 249.26 ±14.93 with highly statistical significant variance (p= <.001 ) between the two groups. Triglyceride in Control group mean ± SD was 137.18 ± 7.93 while in patient group the Triglyceride mean ± SD was 309.02 ± 23.09 with highly statistical significant difference between the two groups. LDL-C in Control group with mean ± SD =was 54.85 ± 7.68 while in patient group, the LDL-C mean ± SD was 133.98 ± 12.26 with highly statistical significant difference between the two groups, as displayed in Figure 1. HDL-C in control group mean ± SD was 58.08 ± 5.28 while in patient group, the HDL-C mean ± SD was 34.48 ± 4.19 with highly statistical significant difference between the two groups.
Figure 2 depicts the CRP test results among the study population. CRP in control group mean ± SD was 5.18 ± 1.08 while in patient group the CRP mean ± SD was 22.23 ± 2.7 with highly statistical significant difference between the two groups.
Table 3 summarizes the plasma miRNA- 29a and miRNA-146a expression levels among the study population. miRNA-29a expression level in Control group mean ± SD was 1.01 ±0.08 while in patient group the miRNA-29a expression level mean ± SD was 2.78 ± 0.24 with highly statistical significant upregulation in the patient group (p= <0.001). miRNA-146a expression level in Control group mean ± SD was 0.94 ± 0.06 while in patient group, the miRNA-146a expression level mean ± SD is 1.92 ± 0.11 with highly statistical considerable variation (p= <.001 ) between the two groups.
Table 4 indicates carotid intimal media thickness (CIMT) among the study population. CIMT in Control group mean ± SD was 0.63 ± 0.24 while in patient group, the CIMT mean ± SD was 1.35 ± 0.14 with highly statistical considerable variation (p= <.001 ) between the two groups.
Figure 3 illustrates Pearson’s correlation coefficients (r) between CIMT and miRNA 29a and miRNA 146a. Regarding miRNA-29a correlation coefficient (r), between CIMT and miRNA-29a expression level was 0.851 with a strong positive relation between the two variables. Concerning miRNA-146a correlation coefficients(r) between CIMT and miRNA-146a expression level was 0.871 with a strong positive association between the two variables.
Figure 4 demonstrates Pearson’s correlation coefficients (r) between CRP test and miRNA 29a and miRNA 146a. The correlation coefficient (r) between CRP test and miRNA-29a expression level was 0.963 with a strong positive relationship between the two variables. r between CRP test and miRNA-146a expression level was 0.954 with a strong positive relationship between the two variables.
Table 5 and Figure 5 show sensitivity, specificity and cut of value of miRNA 29a for CIMT in patient group only. Regarding miRNA-29a, AUC was 0.732, Cutoff value was 2.725, Sensitivity was 74.4%, and Specificity was 72.7%. Regarding miRNA-146a, AUC was 0.812, Cutoff value was 1.955, Sensitivity was 66.7% and Specificity was 81.8%. Regarding Combined miRNA 29a and miRNA 146a, AUC was 0.866, Cutoff value was 4.685, Sensitivity was 74.4%, and Specificity was 100%.
Discussion
Atherosclerotic heart diseases for instance ischemic heart disease and stroke are a main health problem globally. It is a necessity to diagnose atherosclerotic cardiac diseases at initial stages. Recently, microRNAs (miRs) are small RNA capable of binding particular segments on target mRNAs, thus modifying gene expression [14,15].
Carotid atherosclerosis (CAS) is the primary reason of brain stroke. Papers have demonstrated that the degree of carotid stenosis increases the risk of stroke. The rupture of vulnerable plaques in brain vessels is the major process of brain stroke [16].
MicroRNAs (miRNAs) are a set of short, endogenous, single-stranded RNA fragments [17]. Recent studies showed that miRNAs are strongly connected to the incidence and advance of heart diseases, leukemia, DM, and additional diseases [18].
Research have clarified that microRNAs control numerous cell behaviors linked with atherosclerosis progress, varying from hazard factors, to plaque start and advance, to plaque rupturing [19] (Giral et al., 2016). Significantly, prior research has showed that miR-29c and miRNA146 might have a comprehensive and vital role in atherosclerosis development [20].
The objective of this research is to measure Plasma miR 29a and miRNA 146a expression level in patients with carotid atherosclerosis and its correlation with carotid intimal thickness, Our study demonstrated that there was no statistical significant variance regarding age and gender. Regarding hazard factors among the study population, there was a highly considerable variance between the groups under study concerning smoking, hypertension, diabetes mellitus and family history (p= <0.001) that were higher in cases than control group.
In agreement with our study, Liu et al. (2017) collected 170 members and they were allocated into double groups as control group and atherosclerosis patient group [20]. There was no significant variation between the 2 groups concerning gender and age. Similarly, Huang et al. (2018), Huang et al. (2020), Li et al. (2021) and Zhu et al. (2022) proved that there was no considerable variance in age, sex between the carotid atherosclerosis (CAS) and control groups [21-24].
In addition, Wang et al. (2021) showed that participants with ischemic stroke (IS) caused by atherosclerosis were more expected to be smokers [25]. Likewise, IS patients had a greater incidence of hypertension and IHD. However, they were older and mainly males compared with those without IS. This difference may be due to their large sample size. In addition, Huang et al. (2020) found that hypertension was risk factors for CAS caused by atherosclerosis [21].
In the current study, fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), and LDL-C were significantly greater in patient than control group while HDL-C was significantly lesser in cases than control group.
In accordance with our study, Huang et al. (2020) demonstrated that elevated LDL-C values were hazard factors for CAS caused by atherosclerosis, while elevated HDL-C values are protecting factors for CAS [21].
Also, Zhu et al. (2022) revealed that the CAS patients had elevated levels of LDL in comparison to the healthy individuals [24]. Besides, there was no weighty variance regarding other indicators among the two groups, involving FBG, TC, TG, and HDL.
However, Huang et al. (2018) showed that FBG, TC, HDL-C, LDL-C, and TG were not statistically different between the groups [22]. The same findings were shown by Li et al. (2021). This difference may be because their cases were asymptomatic atherosclerosis (AS), while patients having a history of IHD, stroke, diabetes mellitus, heavy smokers, malignancy, and acute heart failure were excluded [23].
In the current study, there was significant variance between both groups as regards CRP that was higher in cases than control group.
In line with our results, Li et al. (2021) showed that it was demonstrated that the AS patients were considerable linked with the CRP in comparison to the healthy volunteers [23]. Furthermore, Huang et al. (2018), Zhu et al. (2022) revealed that the CAS cases had significantly elevated levels of CRP [24].
The results of Huang et al. (2020) study showed that the values of inflammatory indicators in CAS stenosis patients were greater than normal controls, thus signifies that the inflammatory response has a vital task in the CAS incidence [21]. Pathological study has demonstrated that CAS showing features of cells of inflammation are a vital component of CAS plaques [26]. In 1999, Ross obviously revealed that arteriosclerosis is the generation of a reaction of inflammatory process in the intima [27].
Therfore, CAS has progressively been identified as a chronic inflammatory disorder that involves endothelial cell disfunction and enhancing phagocytosis by phagocytes and consequently their conversion to foam cells. Likewise, the inflammatory reaction can as well be approved [28].
In the present study, there was highly statistical significant upregulation of plasma miRNA-29a expression in the patient group than control group. In agreement with our study, Liu et al. (2017) showed that atherosclerosis group had higher miR-29a levels than control group [20]. Also, Huang et al. (2018) showed that miR-29c were markedly variable. The patient group revealed greater miR-29c comparative values, in comparison with the control group [22].
Recently, microRNAs (miRs) are involved in nearly each part of cell or biological courses, and variation of miRs associated with heart diseases, such as atherosclersis [29]. The miR-29, modifying mRNA amount of collagen and inflammatory response shows a complex role in tissue remodeling and vascular injury [30].
It was informed that miR-29 was elevated in the heart tissue next to a myocardial infarction (MI). Circulatory miR-29a levels might signify a novel indicator that significantly correlated with cardiovascular disorders [32]. Furthermore, a former study revealed that miR-29b was included in vascular smooth myocyte migration and multiplication by facilitating an epigenetic effect linked to atherosclerosis [33]. Similar to miR-29b, miR-29a might add to vessel atherosclerosis.
Previous studies have demonstrated that target genes of miR-29 were vital in the generation of atherosclerosis [2,34]. The use of miR-29 antagonists might avoid plaque formation [35]. Also, a previous research showed that the miR-29a expression lead to down-expression of scavenger receptor A and LDL uptake, a vital pathway involved in atherosclerosis [36]. Thus, it can be considered to possess a role in atherosclerosis progress.
In addition, miR-29a may enhance angiogenesis by affecting HMG box-including protein-1 [37] or phosphatase gene [38]. These studies propose that miR-29a might possess a role in atherosclerosis.
In the current study, there was highly statistical considerable upregulation of plasma miRNA-146a expression in the patient group than control group. In agreement with our study, Huang et al. (2020) demonstrated that the values of miRNA-146a in the plasma of the CAS cases were greater than normal group [21]. Besides, the values of miRNA146a were significantly higher with cumulative grades of CAS stenosis. Moreover, the value of miRNA-146aα of the stable atheromatous plaque group was markedly lesser than those with impeding rupture atheromatous plaque [21].
miRNA can be steadily expressed in plasma and used as a biomarker of different diseases. miRNA-146a has been revealed to adjust inflammatory reaction, that are linked with the CAS mechanism [39].
Luo et al. (2017) further revealed that miRNA-146a was incorporated in the generation of atherosclerosis and it was positively related with the intensity of atherosclerosis, in agreement with our study [40].
In the present study, CIMT was markedly higher in patient group than control group. In accordance with our study, Wang et al. (2021) showed that the mean (SD) of CIMT was higher among cases than controls [25]. Furthermore, Li et al. (2021) showed that CIMT would be an indicator of subclinical atherosclerosis patients’ standalone of age, sex, and other hazard factors, and can be used in the early diagnosis of atherosclerosis [23].
In our study, there was a strong positive association between CIMT and each of miRNA- 29a and miRNA-146a. This was in accordance with Liu et al. (2017) who demonstrated that the miR-29a levels were positively correlated with CIMT [20]. miR-29a was revealed to cause atherosclerosis. Greater plasma miR- 29a values may augment the hazard of atherosclerosis. Also, Huang et al. (2020) presented a statistically considerable positive relation between miRNA-146a and CAS stenosis [21].
Furthermore, Li et al. (2021) reported that serum miR-488 level demonstrated a positive relation with CIMT in atherosclerosis patients [23].
Huang et al. (2018) revealed that CIMT was positively related with miR-29c .Moreover, multiple linear regression demonstrated that CIMT was positively related with miR-29c. Independent for age, BMI, blood pressure, and lipid profile, CIMT was still correlated with miR-29c [22].
Furthermore, our results found that there was a strong positive correlation between CRP and each of miRNA-29a expression level and miRNA-146a expression level. This result was in concordance with [22]. Also, Huang et al. (2020) revealed that miRNA-146 was significantly positively correlated with inflammatory markers [21].
In the current study, regarding miRNA-29a, AUC was 0.732, cutoff value was 2.725, sensitivity was 74.4% and specificity was 72.7%. Regarding miRNA-146a, AUC was 0.812, cutoff value was 1.955, sensitivity was 66.7% and specificity was 81.8%. Regarding combined miRNA 29a and miRNA 146a, AUC was 0.866, Cutoff value was 4.685, Sensitivity was 74.4%. and Specificity was 100%.
In agreement with our study, Huang et al. (2020) mapped the ROC curve to test the predictive value of miRNA 146a for vulnerable plaques in carotid atherosclerosis (CAS). It showed a good predictive power with AUC = 0.64.
Also, Li et al. (2021) showed that ROC curve signified that miR-488 possessed a great diagnostic importance in AS patients with 77.6% sensitivity and 99.2 % specificity [23] .
Amr et al. (2018) found that the AUC of miRNA-126 in coronary artery disease (CAD) diagnosis was 0.97 [41]. This high predictive significance of miR-126 for CAD might be justified by its critical tasks in the intervention of endothelium events and inflammatory reaction [42]. Besides, they can be considered as potential indicators for the identification and prognosis of CAD cases, using AUCs of 0.932 and 0.936, correspondingly [43,44].
Conclusion
The findings of this study showed that the combined sensitivity and specificity of miR- 29a and miR-146a in the diagnosis of carotid atherosclerosis are of beneficial value. These results might lead to the improvement of new strategy for the avoidance and treatment of ischemic stroke.
Limitations
Future studies with larger sample size are required and the economic cost of miRNA should be considered.
Acknowledgements
The authors are grateful to all participants in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
HF shared in typing the manuscript and statistical analysis. NS,KE and RYH aided in typing and selecting subjects. HF, HEMS and HS aided in lab investigations.
Conflict of Interest
No relevant conflicts of interest.
Orcid:
Heba Fawzy*: https://orcid.org/0000-0002-9497-1662
Huda E.M.Said: https://orcid.org/0000-0003-1645-2127
Hend Sameh: https://orcid.org/0009-0007-1347-9398
----------------------------------------------------------------------
How to cite this article: Heba Fawzy, Nermin Saad, Khaled Ahmed Elbanna, Huda E.M.Said, Rania Yehia Helal, Hend Sameh, Plasma levels of miR-29a and miR-146a in relation to carotid intima media thickness measurement for detection of carotid atherosclerosis. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(10), 1496-1511. Link: https://jmpcr.samipubco.com/article_195242.html
----------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
