Document Type : Original Research Article
Authors
- Hayam M.A. Mostafa 1, 2
- Mai Raslan 1
- Ahmed Khairalla 3, 4
- Medhat Abd El Fattah 5
- Ahmed O. El-Gendy 3
1 Biotechnology and Life Sciences Department, Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University, 62511, Egypt
2 Beni-Suef University Hospitals, Faculty of Medicine, Beni-Suef University, 62511, Egypt
3 Microbiology and Immunology Department, Faculty of Pharmacy, Beni-Suef University, Egypt
4 Department of Biology, McMaster University, Hamilton, Ontario L8S 4K1, Canada
5 Department of Botany and Microbiology, Faculty of Science, Beni-Suef University, Egypt
Abstract
Mimusops laurifolia (The Tree of Life, as it was known by the ancient Egyptians) belongs to the Mimusops genus, which has been used in folk medicine for a very long time. Recently, it has been concluded that a crude extract containing the saponins from the leaves of M. laurifolia has promising antifungal activity against Candida albicans. This fungus is a widely known opportunistic pathogen linked to systemic and chronic infections. As a result, the current study aims to investigate the anti-virulence potential of these saponins extract against C. albicans at the molecular level in an attempt to better understand how these saponins might function as an antifungal agent against C. albicans. Real-time PCR was used to quantify the relative gene expression levels of critical virulence factors in C. albicans cells treated with saponins versus untreated cells. The potential of these saponins as antifungal agents is likely to be attributed to their gene-regulatory activity, as the expression of some key genes in C. albicans (involved in invasion, survival, and adhesion, such as SAP3, SAP5, and ALS3) was strongly downregulated in saponin-exposed Candida cells. Generally, these data show that M. laurifolia-derived saponins may be efficient inhibitors and virulence modulators of C. albicans. Thus, the current study broadens our knowledge about saponin's potential antifungal properties. Moreover, our discovery of a strong suppressor that acts specifically on virulence-related genes paves the way to develop an oriented class of antifungal drugs.
Graphical Abstract
Keywords
Introduction
Candida albicans is a common member of the human microbiota. It is detected normally in the mouth, genitourinary tract, and gastrointestinal tract in most healthy people [1-5]. However, when the host's immune system is impaired as a result of hospital stay, antibiotic therapy, operation, catheterization, or use of prosthetic devices [6], C. albicans can result in infections, which vary from mucosal infections to serious systemic infections [7]. The emergence of pathogenic C. albicans depends not only on host factors, but also on fungal factors [8]. Deficiency in the immune system, epithelial injury, and microbial dysbiosis are examples of host factors [9]. To be pathogenic, microorganisms require to evolve a strategy that allows them to colonize or infect the host successfully [10]. Consequently, most pathogenic microorganisms, including different species of Candida, have established a robust set of virulence determinants and particular mechanisms to help them infiltrate host tissues, develop infectious disease, and escape from host immunity. Candida species, particularly C. albicans, may express a multitude of virulence factors, depending on the type of infection (superficial or systemic), the infected area of the body, the phase of infection, and the nature of host defense [11].
In contrast to highly specialized pathogenic microorganisms that mainly express a single key virulence factor (for example, C. tetani), the pathogenic C. albicans can express numerous factors that participate in virulence and stabilization of infection, especially in immunocompromised hosts [12].
Despite the fact that C. albicans has a diversity of hydrolytic enzymes [13], secreted aspartyl proteinases (Saps) are the most strongly linked to virulence [11].
Several studies have extensively discussed and emphasized the significant role of Saps in the emergence of C. albicans-related infections. They facilitate the C. albicans pathogenicity through invasion of the host tissue and by destroying the cell membrane. They can also enable the pathogen to skip the defense mechanisms of the host's immunity by attacking its cells. It was further suggested that Saps are involved in the attachment of pathogenic C. albicans to the tissue cells, which is considered as an initiative phase for pathogenesis [14].
In spite of the fact that biofilm formation is most likely the best-known virulence factor of C. albicans, the expression of secreted aspartyl proteinases (SAPs) has been extensively researched as a significant virulence factor [15].
Several studies have found that: (i) the deletion of SAP genes from C. albicans results in a less virulent strain than the original one, and also (ii) inhibitors of that protease result in attenuated C. albicans with less virulence [16].
Secreted aspartyl proteinase enzymes are typically found in the majority of Candida species, and they are expressed by at least ten genes (i.e. SAP1–SAP10) [17].
Moreover, Shi et al. reported that some genes had been known as biofilm formation determinants, and pointed out that the adhesin Als3p (agglutinin-like sequence 3) is a Hypha-specific cell wall protein [18].
It was also clarified that Als3 supports the attachment of pathogens to the endothelial cells of the human umbilical vein and the oral epithelial cells [19].
- albicans' ability to attach to and colonize various types of tissues has been shown to increase its pathogenic potential [20].
Some antifungal medications can act against virulence determinants [21].
However, the rising incidence of the development of antifungal resistance after long-term treatment has evoked researchers' interest in finding new antifungal drugs [22].
As a result, continuing to investigate natural materials (such as plants) for antimicrobial action is critical in the search for new medicinal drugs [23].
According to the World Health Organisation (WHO), medicinal plants could be the best source for receiving various medications. According to Manandhar et al., numerous medicinal plants are significant sources of natural antibacterial and antifungal agents [24].
Mimusops laurifolia (M. laurifolia) has been mentioned in Egyptian historical records since the Eighteenth Dynasty and was known as the "Tree of Life". Its evergreen leaves commonly existed with Egyptian mummies and were discovered in king Tutankhamun's tomb [25], Likewise, M. laurifolia is extensively dispensed throughout the Arabian Peninsula and is the most significant plant species in the Arabian distinct due to its glamorous ethno-botanical history [26].
It is regarded as a medicinal plant and continues to be widely used by locals in southwest Saudi Arabia for a variety of medical purposes [27].
- laurifolia is a member of the Mimusops genus, which is well-known in Indian traditional medicine as a source of fever reducers, astringents, laxatives, and stimulant agents [28,29]. It is worth mentioning that Mimusopus elengi is a well-known plant in traditional herbal medicine, with its leaves being used to cure various bacterial diseases [30].
Mostafa et al. have stated that saponins extracted from M. laurifolia leaves exhibit a promising antifungal action against C. albicans [31].
Therefore, we hypothesised that these saponins may exhibit this action through affecting specific virulent genes in C. albicans at the transcriptional level.
As far as we know, no other investigations have been done on the anti-candida activity of saponins extracted from M. laurifolia leaves, particularly on the molecular level. Therefore, we have been motivated to continue our previous research and conduct this pioneer research to investigate more deeply how these saponins could work as an antifungal agent against C. albicans.
Experimental
Plant material
The Egyptian Museum Garden in Cairo, Egypt, is the source of Mimusopus laurifolia (Forssk.) Friis. Prof. Abdel Halim Abdel Motjale, Chief Researcher and Head of the Flora and Phytotaxonomy Research Department, Agriculture Museum, Giza, Egypt, verified the plant's authenticity. The Plant List website (http://www.theplantlist.org) was used to verify the plant's name. The sample was consigned in the Faculty of Postgraduate Studies for Advanced Sciences, Biotechnology and Life Sciences Department, Beni-Suef University, Egypt, with the spicemen voucher number (PSAS-BT01) [31].
Preparation and usage of crude saponins
In brief, the collected leaves were dried and milled into a fine powder, then 80 g of the powdered leaves were mixed with 350 ml of 80% methyl alcohol and left for two days at room temperature with continuous shaking at the rate of 150 rpm. After filtration of the mixture, the maceration process of the residue was repeated twice to increase the yield. Next, the filtrates were collected and evaporated at 40 °C using a rotary evaporator. Next, the concentrate was freeze-dried using a lyophilizer to produce a yellowish-colored residue.
A volume of 100 mL of distilled water was mixed with the obtained residue before being transferred to a separating funnel and mixed with 100 ml chloroform. The separating funnel was shaken and held for a while to obtain two completely separated layers. The bottom layer containing chloroform was removed. The aqueous solution was extracted several times with chloroform to ensure complete extraction.
The remaining aqueous layer was then extracted with 100 ml of saturated n-butanol. At that time, the aqueous layer was the lower layer. This process was carried out three times. The n-butanol layers were collected and evaporated at 40 °C using a rotary evaporator to get the n-butanol fraction [32].
To improve the saponin yield, 300 mL of cold acetone was added to the n-butanol fraction dissolved in methanol [33].
Finally, the precipitate was then filtered and dried. This precipitate, which represented the crude saponins, was then kept in a sealed container in the refrigerator until required. As reported in our previous work, a saponin froth test confirmed that the obtained precipitate was rich in saponins. In addition, saponins study using HPLC/UV, performed in accordance with the guidelines established by Thalhamer and Himmelsbach [34], confirmed the existence of 15 saponin components [31].
6.4 μg.ml-1 of saponins extract, which represent the minimum inhibitory concentration of the extracted saponins [31], was prepared and sterilized through a Millipore sterile syringe filter immediately before use.
Effect of saponins extract on expression levels of C. albicans virulence genes
To study the effect of saponins mixture extracted from M. laurifolia leaves on the gene expression of some virulent enzymes in C. albicans (SAP3, SAP5, and ALS3), a one-step reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay was carried out.
Briefly, C. albicans ATCC 60193 was obtained from the Department of Microbiology, Faculty of Pharmacy, Beni-Suef University. One colony of C. albicans cultured on Sabouraud dextrose agar plate was sub-cultured in 10 mL of Sabouraud dextrose liquid medium, and the culture was grown for 18 hours at 30 °C to verify the purity [35,36].
As described by earlier studies [37,38], C. albicans were placed in 10 mL of RPMI 1640 (with L-glutamine) to obtain a suspension of the desired final concentration of 1 × 106 cells/ml. C. albicans suspension was supplemented with the saponins extract's MIC value (6.4 µg.ml-1) [31] and incubated for 24 hours with brief shaking.
The negative control contained C. albicans cells incubated under identical experimental circumstances but without saponin exposure. Next, the manufacturer's instructions of TRI Reagent® and Direct-zol TM RNA MiniPre kits were followed to isolate and purify total RNA from the centrifugated C. albicans cells.
A one-step kit (HERA SYBR® Green RT-qPCR kit) was used to perform reverse transcription of the total RNA to prepare cDNA templates and perform qPCR analysis. RT-qPCR was performed following the manufacturer's instructions. Primer sequences of the investigated virulence genes are presented in Table 1.
As an endogenous control, the housekeeping gene ACT1 was utilized. The relative gene expression of each targeted virulent factor was compared to the corresponding one of untreated C. albicans cells (expression = 1). The formula 2-ΔΔCT was used to calculate the results. Each assay was independently conducted three times to obtain the mean value. The expression change of each investigated gene was displayed as a fold change in expression. In comparison to the control samples (untreated cells), the results show a logarithmic fold increase.
The following equation was used to determine the relative fold change: Fold change = .
The mean CT (cycle threshold) value of the house keeping gene (ACT1) was subtracted from the target gene to calculate its normalized CT value, represented as ΔCT, and then to determine the ΔΔCT value, the calculated ΔCT of the gene of interest in the control sample (untreated cells) was subtracted from the ΔCT value of the corresponding gene in the saponins treated cells (test sample).
Statistical analysis
Every experiment was carried out at least three times, with assay values represented as means ± SD. GraphPad Prism software was utilized to perform the statistical analysis. All comparisons were made using the Kruskal-Wallis test, followed by the Dunn test.
Results and discussion
In an attempt to learn more about the anti-virulence activity of saponins extracted from M. laurifolia leaves and how they can inhibit C. albicans growth, we examined the expression profiles of certain key adhesion, invasion, and survival genes.
Real-time PCR was conducted to test the saponins mixture for possible activity against certain virulent genes in C. albicans ATCC 60193, in an effort to further understand its antifungal mechanism.
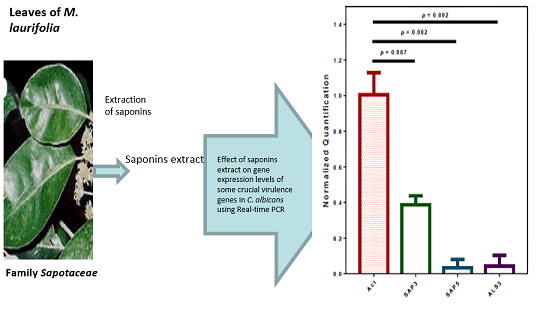
A total of three crucial virulence-related genes in C. albicans were investigated, namely, SAP3, SAP5, and ALS3. Following saponins treatment, the three tested genes had lower mRNA levels in the cells treated with saponins than in the control group.
Saponins significantly suppressed the expression of the SAP3, SAP5, as well as ALS3 genes by 61% (p = 0.002), 97% (p = 0.002), and 96% (p = 0.007), respectively, according to our real-time PCR (qPCR) results (Figure 1, Table 2).
The illustrated fold changes in gene expression are relative to the control group's results (C. albicans ATCC 60193 not exposed to saponins mixture). In addition, the expression data were normalized using the housekeeping gene ACT1. The results are displayed as the means of three separate assays (Figure 1, Table 2).
All of the genes that have been downregulated play important roles in the pathogenicity and virulence of C. albicans ATCC 60193. Aspartyl protease enzymes (encoded by the SAP3 and SAP5 genes) are fungus-secreted enzymes that may facilitate infection through catalyzing the hydrolysis of host cell membrane components [14].
The ALS3 gene belongs to the ALS gene family that is required for C. albicans adherence [41].
Candidiasis is getting more prevalent throughout the world [42].
Doubtless, virulence factors enhance a microorganism's ability to establish itself in or within a host as well as its ability to cause disease [43].
Targeting virulence factors and the genes that encode them to disarm pathogens is a promising approach for preventing and curing infectious diseases [44,45].
Rather than direct biocidal activity, one of the current paradigms in developing novel antimicrobial medicines is to target the microorganism's virulence factors [46,47].
For decades, medicinal plants have participated in improving health and treating some particular ailments [48]. It has been reported that natural products such as plants give the possibility to develop novel drugs as a result of having various chemical compounds [49].
According to 111 scientific documents released between 1969 and 2015, C. albicans exhibited susceptibility to 142 natural compounds [50].
- laurifolia (Forssk.) Friis, of the family Sapotaceae, is a native species confined to the Red Sea Mountains and the Gulf of Aden [51].
It is considered as a medicinal plant and is still commonly used by the locals in Saudi Arabia for various therapeutic uses [27].
It was discovered that their leaves contained fifteen triterpenoidal saponins [52].
Recently, saponins have attracted more attention because of their unique chemical composition and biological properties. Triterpenoid saponins, which are found in a variety of higher plants, have a variety of pharmacological actions, including antibacterial and antifungal activities [53].
Since saponins extract in our previous study showed a notable antifungal efficacy against C. albicans with MIC value of 6.4 μg.ml-1 and the molecular docking analysis and molecular dynamic simulation of the investigated saponins showed their affinity toward the docked enzymes, especially SAP3 and SAP5 [31], we hypothesized that these saponins may also affect some virulent genes in C. albicans at the transcriptional level. The current work demonstrated the value of using saponins to address some genes that code for virulent components. Several studies have already reported the role of genes investigated in the current study in the virulence of C. albicans [14-16,20,54].
Eskander et al. stated that the saponins extract of M. laurifolia leaves comprises several components (saponin 1-saponin 15) [52].
Therefore, its antifungal effect could be multitarget. In light of this, C. albicans ATCC 60193 was exposed in RPMI 1640 media to MIC value (6.4 µg.ml-1) of a saponins mixture extracted from the leaves of M. laurifolia. As expected, the findings of real-time PCR revealed that following exposure to the saponin compounds, some important genes related to survival, adhesion, and invasion were indeed differentially expressed.
This study observed a strong effect of saponins extract on the expression of SAP3 and SAP5 genes in C. albicans that was apparently suppressed by 0.61 and 0.97 folds, respectively. Aspartyl protease enzymes are important virulence factors in C. albicans, as they participate in tissue attack, biofilm production, adhesion, as well as phenotypic switching7.
Moreover, a study conducted by Kadry et al. has verified the linkage between reduced sensitivity to antifungal medications and aspartyl proteinase production, as it has been reported that all antifungal resistant isolates were able to produce aspartyl proteinase [55].
Moreover, the saponins mixture sharply inhibited the expression level of the ALS3 gene, which is essential for the fungus to develop biofilm, adhere to, and aggregate with other substances [56].
In fungal infection, attachment to host tissues belongs to the initial stages of the infection process, and inhibiting adherence restrains the colonization and infection [57]. Overall, this genetic assay demonstrated the ability of saponins extracts to attenuate C. albicans pathogenicity via affecting the expression of some fungal master genes.
We suppose that, this work is the first to characterize the anti-virulent activity of saponins derived from M. laurifolia leaves, particularly at the transcriptional level. However, our investigation's findings agree with the findings of some studies conducted to investigate the antifungal mode of action of saponins extracted from different plants.
Regarding the effect of saponins on adherence, a study conducted by Patel et al. showed that the extract of Dodonaea viscosa var. angustifolia, which contains several phytochemical substances including saponins, reduced the attachment of C. albicans to oral mucosa. Moreover, they reported that the investigated extract may reduce the biosynthesis of adhesins and subsequently reduce the attachment of C. albicans to epithelial tissue via affecting ALS genes [57].
Furthermore, as shown in research of various biological activities related to phytochemicals, flavonoids, alkaloids, and saponins are expected to have anti-SAP activity or a synergistic effect that boosts this activity [58].
Conclusion
Our findings revealed that saponins extract of M. laurifolia leaves is a promising and potential anti-candida agent that affects some basic vital processes through inhibition of some key virulence factors of C. albicans. Interestingly, our research revealed a virulence-selective treatment strategy. This research will pave the way for more preclinical and clinical research to inspect the potential of saponins from M. laurifolia as a new natural product-based anticandidal medication. Furthermore, despite the fact that resistance to available antifungals is common, our findings revealed that a saponin mixture extracted from M. laurifolia leaves exhibited antifungal activity against C. albicans by affecting several different virulence strategies of the fungal cells, implying that resistance to these compounds is supposed to be unlikely. As a result, combining saponin with other antifungal agents presently on the market could be a promising strategy for treating resistant C. albicans strains. Consequently, more research into this anticipated synergistic interaction as well as the potential of combination therapy may provide insight.
Acknowledgements
The authors thank Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University and Faculty of Pharmacy, Beni-Suef University, for all supports.
Authors’ contributions
Mai Raslan, Ahmed S. Khairalla, Ahmed O. El-Gendy, Medhat abd El Fattah, and Hayam M. A. Mostafa designed the research. Mai Raslan, Ahmed O. El-Gendy, and Hayam M. A. Mostafa conducted the research. Ahmed O. El-Gendy, and Hayam M. A. Mostafa analyzed the data. All authors contributed to writing and finalizing the manuscript.
Conflict of interest
There is no conflict of interest to declare in this article.
Orcid:
Hayam M. A. Mostafa: https://orcid.org/0000-0003-1847-2308
Mai Raslan: https://orcid.org/0000-0003-3136-6833
Ahmed S. Khairalla: https://orcid.org/0000-0003-1204-1478
Medhat.abd El fattah: https://orcid.org/0009-0009-1409-0354
Ahmed O. El-Gendy: https://orcid.org/0000-0002-0980-5185
-----------------------------------------------------------------------------
How to cite this article: Hayam M.A. Mostafa*, Mai Raslan, Ahmed S. Khairalla, Medhat.abd El Fattah, Ahmed O. El-Gendy. Saponins from Mimusops laurifolia target some key virulence factors in Candida albicans. Journal of Medicinal and Pharmaceutical Chemistry Research, 2023, 5(10), 895-906. Link: http://jmpcr.samipubco.com/article_177583.html
-----------------------------------------------------------------------------
Copyright © 2023 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
