Document Type : Review Article
Authors
Physics and Mathematics, Institute of Engineering and Technology, Universidad Autónoma de Ciudad Juárez, Juarez, Chihuahua, Mexico
Abstract
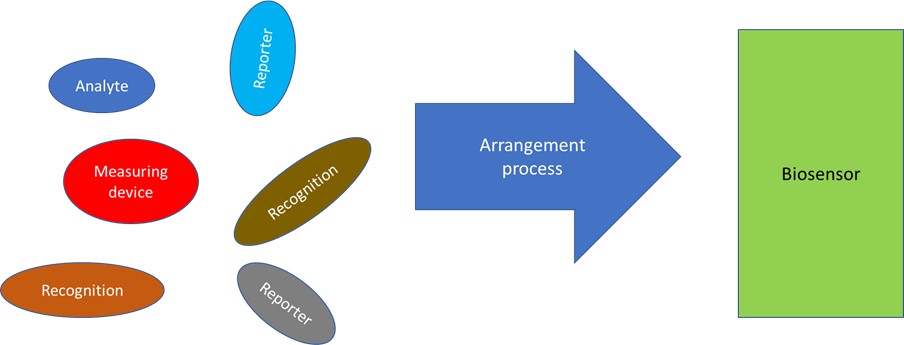
The basic elements in biosensors are the recognition element, reporting element, and measuring device. The typical structure for the biosensing process to occur is the one where the recognition element is first in the process, followed by the reporting element, and finally the measuring device. This simple structure, however, can be arranged in many ways, therefore creating different biosensors e.g., ELISA, lateral flow, or electrochemical. This work is a review from 2009 to 2023 of the literature focusing on describing the biosensor elements and their arrangements as well as describing their functions for several known biosensors. There is also a description of how the different arrangements affect the biosensor performance. The analysis is done independently of the different analytes, biosensor materials, or their fabrication. One objective is to inspire new biosensors by looking at structural changes in contrast with focusing on improving a synthesis, changing a concentration, or changing a material substrate. This work also aims at providing a synthetic framework to contribute to the understanding of a large amount of literature available in this field.
Graphical Abstract
Keywords
Introduction
In 2022, the worldwide biosensors market reached a significant valuation of USD 26.8 billion, with experts projecting a steady compound annual growth rate (CAGR) of 8.0% from 2023 to 2030. This remarkable growth is primarily fueled by the escalating demand for biosensors, driven by their diverse range of applications [1]. Beyond their impressive biosensor applications, the study of biosensors often overlooks the intricate interplay between their essential elements. While the analyte, recognition element, reporter, and measuring device are individually recognized for their contributions, the true significance lies in their synergistic collaboration. It is in this often-missed interaction that biosensors transcend from mere detectors to extraordinary diagnostic tools and environmental monitors. Therefore, focusing on describing the biosensor elements and their arrangements, as well as understanding their functions for several known biosensors, is considered of great relevance in unraveling the full potential of these advanced devices. The following are a few areas where biosensors [2] are used: detection of illegal growth proteins in meat [3], air monitoring for pollutants [4], the efficiency of antibodies in vaccination [5,6], identification of antigens in clinical studies, and detection of tumor markers in treatment development [2,7,8]. A basic biosensor is a system composed of the following elements: an analyte, a recognition element, a reporter, and a measuring device [9]. The biosensor can detect analytes like proteins, molecules, DNA, microbes, bacteria, and viruses [10]. The biosensors can be classified by their detection principle in two branches affinity and chemical [9,11]. In the affinity branch, there is the immunoassay and the immunosensor which use the affinity process between the analyte and the recognition element to determine the presence and amount of the analytes of interest [9,11-14]. The ELISA (Enzyme-Linked Immunosorbent Assay) is an example of an immunoassay [4,7-10]. In the chemical branch, the analyte is detected and quantified using the chemical (enzyme) transformation that is taking place between the analyte and the recognition element. [17]. The home glucose monitor is a chemical biosensor [18]. The structure formed by the analyte, recognition element, reporter, and measuring device can affect the performance of the final biosensor. For example, if the recognition element and the reporter instead of being separated are fabricated together as one piece, they will then form what is called an immunosensor, and this structure enables a small biosensing device [19,20]. For this review, it shall be understood as the biosensor structures the arrangements of the analyte, recognition element, reporter element, and measuring device. In the literature, the structures formed by these elements are frequently depicted in tables or schematic figures [10,18,21], where sometimes there is no clear identification of the basic elements or functions that make up the biosensor. This review, however, synthetizes several structures, using the basic biosensor elements as well as relates them to their specific performance properties like the limit of detection, dynamic range, or others. There are other reviews on biosensors, focused for example, on how they use graphene and carbon quantum dots [10], their use of flexible materials [8], or the biosensors developed for specific analytes [2,7,22], or by the material used in their labels [23-25], in summary mainly focusing in the elements themselves. On the systemic view, there are other works on ontologies e.g., for the design of the recognition element in biosensors by finding appropriate aTF molecules by searching in molecules databases [26], or the representation of knowledge on regards environmental risks for the determination of what analytes to monitor using biosensors [27], on AI biosensing, e.g., AI wearable biosensors, and IoT networks [28], AI on continuous glucose monitoring [29], or on systems feedback concepts e.g., applied on gene circuits in biosensors [30,31]. However, we could not find biosensor reviews based on elements and their arrangements.
In this review the following topics will be discussed, biosensor performance requirements, biosensor structure elements, elements used in biosensors, biosensor structures, opportunities, and challenges in the study of biosensor structures, and finally the conclusions. This work provides a status on the biosensor structures and suggests combinations for new ones, which can be of interest for the expert, or novice working in this field.
Biosensor performance requirements
It is often found in the literature as the biosensor’s performance requires the limit of detection (LOD), the range of measurement, and less frequently the sensitivity [31]. These three are used to compare performance among different biosensors. The limit of detection is the smallest quantity of analyte that can be detected. The range of measurement is the difference between the smallest and largest quantity of analyte that can be measured. The sensitivity is the slope on the calibration line. This slope indicates how much analyte is required to measure a given analyte concentration value. It is less frequent to report performance variables like the transducer preparation time, incubation time, long-term stability, and assay time [32]; these variables provide information on the time that takes to perform a test or the time that the elements are stable and usable to perform the test. Other performance items that are not frequently reported are precision, accuracy, measurement stability, noise sensitivity, specificity, false positive rate, or false negative rate.
Biosensor structure elements
The biosensors can have numerous classifications [33,34], which include recognition method, analyte, reporting signal, structure, and others (Figure 1). It is important to note that these classifications are not exhaustive, implying that further categorizations are possible based on the specific requirements. Next follows the descriptions, for each of the fundamental biosensor structural elements: the analytes, recognition, reporting, and measuring elements. In Table 1, there are specific examples of analytes and recognition elements while in Table 2, there are examples of reporting elements. Furthermore, in subsequent section, it is elaborated on the construction of synthetic biosensor structures by combining these fundamental biosensor structural elements. For instance, ELISA, electrochemical, lateral flow, and surface plasmon resonance biosensors (Figure 2) are synthesized among others.
Analyte
The analyte can be any molecule or biological compound that is of interest. This biological compound needs a biological recognition element for its detection and quantification. Although the analyte is not fabricated with the biosensor, it should be considered for its design. Therefore, in this work, it is considered one of its elements. The determination of the analyte drives the selection of the recognition element and can be critical for the selection of the biosensor structure [9]. It is also necessary to understand the analyte amount, form, and medium in which will be presented for the interaction to happen with the recognition element. In many biosensors, the analyte is first treated in ways to improve, for example, the biosensors’ sensitivity, specificity, or background noise level. The treatments could include dilution to match the biosensor’s measuring dynamic range [19], or filtering to remove analytes that are not of interest [35], and that could cause background noise or reduce precision.
Recognition element
This element is the one that detects the analyte of interest. This element is selected for each specific analyte. If the analyte changes, the recognition element is almost sure to change. The recognition element is in direct contact with the analyte, either through a sample or immersed in the analyte [13]. Several times, the recognition element is affixed to a solid phase, which permits the trap of the analyte of interest and immobilizes it [12]. Typical recognition elements for immunoassays and immunosensors are antibodies and aptamers [36].
Reporting element
The reporting element provides a signal upon the analyte detection by the recognition element. The signal is the connection between the analyte detection and the measuring device. Sometimes the reporting element needs another piece to complete the generation of the signal for some authors, the reporting element is called a transducer [9] and its function is to change a signal from one form to another. Examples of reporting signals are fluorescent light, voltage, electrical current, mass change, heat, capacitance change, light absorption, color change, or vibration frequency shifts [9, 37]. It is frequent to use enzymes and nanoparticles as reporting elements [38].
Measuring device
The measuring device reads the signal provided by the reporting element. The measuring device uses the signal to generate a reading that is meaningful to the human. Examples of measuring devices are spectrometers, fluorometers, voltmeters, amperemeters, thermometers, and the human eye. The measuring device can be interconnected thru Wi-Fi, Bluetooth, or other information networks, and cell phone applications. The interconnectivity is key for wearable biosensors and artificial intelligence applications [28,39].
Examples of elements used in biosensors
The analytes drive the recognition elements, and the reporters define the kind of signal used in the biosensor. There are thousands of these elements and some examples are presented in Tables 1 and 2. These tables and their arrangement can grow to become large databases useful in the design of biosensors. These tables, however, do not say much about the biosensor structure, the biosensor structures will be discussed later in this work. In the literature, it is frequent to see graphical schemes that describe information on regards the sensor structure. Besides, in these same graphical schemes, it is frequent to find information on how the assays are performed and sometimes information on the synthesis of the elements [40]. Therefore, these graphical schemes can be overcrowded with information and therefore become confusing.
List of recognition and reporting elements
The analyte and signal would be typical inputs in the development of a biosensor. The following tables intend to define elements and their performance using as inputs the analyte (Table 1) and the output signal (Table 2) [41]. The entry column in Table 1 is the analyte, the second column describes the type of recognition element, and the next column provides brief notes on function and performance. For Table 2, the entry column is the signal, and then the description of the recognition element, and finally a description of function and performance. The entries to the tables are not exhaustive since there are thousands of them. The assay sequence or synthesis of the elements is not mentioned in these tables. However, a quick word on regards synthesis is that environmentally friendly methods have been developed for the production [42] of some of these elements.
Biosensor structures
The following structures show how the basic elements can be combined. These structures do not mention which are the materials for each element. By not mentioning the specific materials, the emphasis is on the function to be performed by the element in the given structure. The function description can be used to find alternate materials or geometries, or structural solutions that can also perform as described. These structures are not all the ones possible. The descriptions show the relation of the structure to the biosensor performance as reported in the literature for each case. This understanding can help decide which one to use in the next research or application project. This work can motivate the search for combination rules for the biosensor’s elements among different structures. The combination rules can help design the biosensors. Some other processes are part of a biosensor and are not listed as structural elements, like exciting, mixing, incubating, filtering, vibrating, immobilizing, heating, separating, transporting, storing, diluting, pipetting, centrifuging, catalyzing, and others. Any of these processes are mentioned as required. Therefore, the structures described are synthetic structural descriptions for the biosensors chosen for this work.
ELISA sandwich
The ELISA sandwich immunoassay (Figure 3) has the following elements in its structure: analyte, the recognition element 1, the recognition element 2, recognition 3, the reporting element, and finally the measuring device [12]. The structure is displayed in (Figure 3). The recognition element 1 is attached to the solid phase and the analyte binds to it and is immobilized. The recognition element 2 binds to the analyte, and the recognition element 3 binds to the recognition element 2. The reporter is bound to the recognition element 3. The measuring device reads the signal from the reporter(s) [15]. The functions of the elements related to this structure are the following: The immobilization of the analyte by the affixed recognition element 1 allows for a washing step that will remove from the assay all the material not bound by the affixed recognition element 1, this increases the specificity, precision, and accuracy of the immunoassay. This also reduces the noise from unwanted analytes. The use of a recognition element 2 allows for more than one recognition elements 3, to bind to it. Therefore, recognition element 2 acts as an enabler for signal amplification. A stronger reporting signal is possible since each recognition elements 3 has a reporting element in it. Different analytes can be detected using a different recognition element 1, which is affine to the new analyte. The ELISA immunoassay can have limits of detection of 2.6 ng/ml as reported in [16]. The following articles [50-52] report several applications that use this type of structure, these by no means are all the ones possible.
Lateral flow sensor
The lateral flow sensor (LFS) (Figure 4) has the following elements: an analyte, recognition element 1, recognition element 2, a reporter, and a paper absorption pad. Their locations are critical (Figure 4). The recognition element 1 is a moving element and it is not fixed to the absorption pad. The reporter is bound to the recognition element 1. The recognition element 2 is affixed to the paper pad. The recognition element 1 has the function of carrying the reporter and binding to the analyte.
The recognition element 2 has the function of immobilizing the reporter so that it can be activated by a reagent and generate a color change. The biosensor works [53] by depositing the analyte on one extreme of the paper pad, the analyte moves laterally because of capillarity forces in the absorbing pad while moving laterally the analyte moves over the recognition element 1 and reporter, where they bind together if they are affine. This assembly of the analyte, recognition element 1, and a reporter will travel until it reaches the location of the recognition element 2. The recognition element 2 is affixed to the paper absorption pad and therefore immobilizes the traveling assembly if they are affine. In the area where the recognition element 2 is affixed, there is also a reagent that will activate the reporter. A color change is caused by the activation of the reporter by the reagent, and this is proportional to the analyte concentration. This color change can be seen by the naked eye and will signal a confirmation that the analyte of interest exists in the sample. If no analyte is present in the sample the recognition element 1 and reporter do not bind to the recognition element 2 and continue traveling until the end of the paper pad. Therefore, a color change is not activated. Moreover, this structure allows for easy-to-use tests. This also allows for point- of-care testing. Many analytes tests with LFS are commercially available.
This structure requires an extra measurement device to provide the amount of analyte. The following works show sensors using this structure [54-56].
ELISA sandwich and paper sensor
The biosensor structures can be combined, and one example is the combination of an ELISA sandwich assay and a paper sensor (Figure 5) described in [57]. The paper sensor uses paper to affix a recognition element and generate an optical signal once the recognition element binds to an analyte. The elements on this combined sensor (Figure 5) are the same ones as for the ELISA described before (without a measuring device) plus a recognition element 4 impregnated on the paper sensor (Figure 3). Therefore, the complete elements are the analyte, recognition element 1, recognition element 2, recognition element 3, reporting element, recognition element 4, and the measuring device. The function of the ELISA elements was described before, except for the recognition element 3 which is now used as an ion generator, and the recognition element 4 in the paper sensor which will turn into a reporter by ion exchange. The way the sensor works is that once the ELISA has three recognition elements and reporter as a complete assembly, then this assembly is dissolved in ions. After that, the ions are poured onto the paper sensor and through an ion exchange reaction, recognition element 4 turns into a reporter by fluorescent quenching. Its fluorescence quenched intensity is proportional to the number of ions which respectively are proportional to the analyte concentration. This structure allows for the use of a simpler measuring device like a digital camera, or even the naked eye, instead of using a fluorometer.
Electrochemical sensor
The electrochemical sensor (Figure 6) [9,58] has the following elements: analyte, the recognition element 1, the recognition element 2, reporter, and electrode that functions as a base for the biosensor see (Figure 6). The recognition element 1 is affixed to the electrode; the reporter is bound to the recognition element 2. The function of the recognition element 1 is to capture and immobilize the analyte. The recognition element 2 carries the reporter. The reporter in this case has the function of a catalyzer which will promote the electron interchange with the electrode. The sensor works by capturing the analyte, and then attaching the recognition element 2 and reporter to this immobilized analyte. Thereafter, the reporter promotes the electron transfer to the electrode, and the electrical signal is generated. This structure normally has the advantage of being a small device and normally does not require sample preparation; the sample is directly deposited on top of the electrode [59]. Another advantage is that it provides fast results. It can be used at the point of care therefore not requiring specialized testing locations.
Digital ELISA
The digital ELISA [60] is composed of an analyte, recognition element 1, recognition element 2, reporter, and magnetic bead attached to recognition element 1 (Figure 7). The recognition element 1 and the magnetic bead are free to move. The function of the recognition element 1 and the magnetic bead is to bind with the analyte. The magnetic bead will function as an immobilizer since it will later bind to a magnetic microwell. The recognition element 2 functions as a carrier of the reporter. The reporter function is to send a fluorescent signal to the measuring device. The measuring device counts the number of wells that are emitting fluorescence. The sensor [57] works by mixing the sample with the analyte with a solution of a compound of magnetic beads and recognition element 1. This assembly binds to the analyte, and then the mixed solution is poured onto a base with magnetic microwells. Next, the micromagnetic beads are magnetically affixed to a microwell, one microbead per microwell. Next, the recognition element 2 and reporter attach only to the magnetically fixed assemblies that have analyte on them. Finally, the reporter is activated with the UV radiation and will emit fluorescence. Using a fluorescent microscope, the number of emitting wells is counted. This count directly translates into a proportion of the analyte. This assay has been able to detect amounts of analyte as low as 14 fg/ml, which is extremely sensible [61-62].
Mass or form change
The mass or form change biosensor (Figure 8) has the following elements: analyte, recognition element and surface to attach the recognition element. The recognition element has the function of trapping the analyte and immobilizing it. The attachment surface has the function to hold the analyte and recognition element in place. The attachment surface will also function as a reporter due to the change in the mass caused by the extra analyte mass attached to it by the recognition element.
The sensor works by transforming the change in mass into a change in vibration frequencies, [63] or a weight change used as signals for the appropriate measuring devices. Also, the extra analyte can cause deformation of the surface which can also be measured. One advantage of this method is that it does not require a reporter since the attaching surface will function as a reporter. This type of sensor is sensible; the work in [64] reports a 5.4 ng/ml LOD.
Surface plasmon resonance
This sensor has the following elements: analyte, recognition element, and attaching surface the same structural elements as for the sensor in 4.6. The elements' functions are the same. The elements' difference is not their structure but rather how the reporting signal is produced. In this sensor, the binding of the analyte to the recognition element causes a change in the plasmon wave on the interface between the surface and the recognition element 1. As mentioned earlier, the surface plasmon resonance (SPR) has the advantage of not needing an extra reporter. Furthermore, it can continuously monitor the kinetics of the binding of the analyte to the recognition element. The following review work [65] mentions several of these sensors.
Multiplexing
Multiplexing is the detection of several different analytes mixed in the same sample in the same essay. One multiplexing structure is to form specific areas on the immobilizing surface for each analyte. In this case, the elements and functions are the same as for an ELISA sandwich but for each area, there are different recognition elements. The advantages are the same as for an ELISA [12], but there is the possibility of interference and noise between adjacent areas (Figure 9).
Another possible multiplexing structure is not to select different areas for different analytes but to select different reporters as well as different recognition elements for each different analyte. This structure has more interference problems than the first one. The review work on [66] shows several multiplexed biosensors. This other work uses several carbon nanotube fibers as multiplexing areas [67] instead of a continuous flat surface divided into different analyte detection areas. The recognition elements are affixed to the cylindrical surface of the carbon nanotubes.
AlphaLisa
The AlphaLisa has the following elements: analyte, recognition element 1, and recognition element 2. None of the elements is immobilized (see Figure 10). This is a homogeneous assay. It means that there is no removal of the unwanted analytes. The sensor works [68] by the analyte binding to the recognition element 1, and then this compound binds to the recognition element 2. The proximity of the two recognition elements creates a chemiluminescence event that is used as the reporter. The most significant advantage of this type of essay is that it does not require washing for the removal of the unwanted analytes; this simplifies the assay [69].
Reporter cluster disaggregation
This sensor has the following elements: the analyte, the recognition element 1, the recognition element 2, and a cluster of reporters. The recognition element 1 has a magnetic particle on it, and recognition element 2 has a catalytic element on it see (Figure 11). The work in [45] shows a sensor of this type. The sensor works by the analyte attaching to the recognition element 1 and its magnetic particle, as well as attaching to the recognition element 2 and its catalytic element, and then this assembly is separated from the rest of the sample by employing the magnetic particles on recognition element 1. Next, this assembly is mixed in a solution with the clusters of reporters. The catalytic element in the recognition element 2 causes the reporter cluster to disaggregate this, therefore, causes a change in the optical density of the solution. The change in optical density is proportional to the concentration of the analyte of interest.
The approach can also be reversed and look for aggregation of elements [70].
Target-induced structure switching (TISS)
This sensor structure has the following elements: An analyte and a recognition element. The recognition element is not affixed; therefore, this is a homogeneous assay since there are no washing steps (Figure 12). The sensor works by mixing the analyte and the recognition element, once the recognition element and the analyte bind then the recognition element will change its structure. The change in the structure of the recognition element 1 creates a signal [71]. These are sensible assays as shown in work [72] and they are simple to do. The assays described in the mentioned work took 1 minute to complete.
Thermal
The thermal biosensor has an analyte, a recognition element, and a measuring device. The sensor works by the analyte interacting with the recognition element; this causes a thermal signal that the measuring device reads. This sensor has problems with selectivity. The following reference [73] lists several of these biosensors.
Opportunities and challenges in the study of biosensors structure
There are currently thousands of papers related to biosensors for a similar amount of analytes [41]. The basic structure made of an analyte, a recognition element, a reporter, and a measuring device is not enough to classify the many different biosensors out there. The structure of the biosensor needs to be sometimes extracted from tables and graphical summaries. This can overwhelm a newcomer in the field. Therefore, reviews that focus on the biosensor's structures and the functions of their elements in each structure are necessary. Also, there is a need to enlist more performance items to have a better comparison base between biosensors. Performance items that can help in the previous objective are precision, accuracy, measurement stability, cost, ease to use, ease of assembly, point of care ready, cost, specificity, and others. There is further need to formalize how to measure some of the previous items like “easy to use”. Likewise, there is a challenge in trying to define rules for the combination of elements among different structures. Rules of combination across different structures can facilitate biosensor design [33].
There is another challenge in standardizing the use of terms. An example is biosensor, it is sometimes understood that this term means an essay, or it can also mean a device. The terms biosensing, immunosensor, immunoassay, bioassay, biosensor, and bio-detection are sometimes used as synonyms which can lead to confusion. In this work, the term biosensor is used as a general term for detecting by any means biological analytes. Biosensor databases exist, an example is in [74], which is a web-based database dedicated to fluorescent biosensors, there are also data sets available to train machine learning and AI applications [75]. However, there is a challenge to standardize the information framework used in these databases.
Structural design of biosensors
One example of biosensor development using the previous analysis is combining target-induced structure switching (TISS) and multiplexing, therefore obtaining a biosensor structure of multiplexed TISS, and at the same time, having the possibility of detecting several analytes in the same solution. Another structure example is doing homogeneous and heterogeneous essays simultaneously with different recognition elements for the same analyte on each essay, this would allow for increased sensitivity and specificity. The previous structures might not be technologically possible now or maybe impossible, but the analysis shown in this work allowed discovering combinations like these that could inspire new research
Conclusion
The significant advances in the development of biosensors, particularly through the lens of structural vision are presented in this work. By describing the specific functions required for each element within various biosensor structures, it enables to identify and pursue different implementation possibilities for biosensors, fostering innovation and advancements in the field.
Conflict of Interest
The authors declared that they have no known conflicting financial interests or personal relationships that could have influenced the work reported in this article.
Acknowledgments
The authors thank the CONAHCYT for the PhD scholarship awarded to Jorge Lopez.
Orcid:
Jorge L. López-Dino: https://orcid.org/0000-0002-0907-8547
Juan Francisco Hernández Paz: https://orcid.org/0000-0002-1621-835X
Imelda Olivas Armendáriz: https://orcid.org/0000-0003-2233-0310
Claudia A Rodríguez González: https://orcid.org/0000-0002-1820-1893
-------------------------------------------------------------------------------------
How to cite this article: Jorge L. Lopez-Dino*, Juan F. Hernandez-Paz, Imelda Olivas Armendariz, Claudia A. Rodriguez Gonzalez. Structural design of biosensor: A review. Journal of Medicinal and Pharmaceutical Chemistry Research, 2023, 5(10), 915-934. Link: http://jmpcr.samipubco.com/article_177586.html
-------------------------------------------------------------------------------------
Copyright © 2023 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
