Document Type : Original Research Article
Authors
1 Clinical Microbiology Study Program, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2 Department of Clinical Microbiology, Faculty of Medicine, Universitas Airlangga – Dr. Soetomo Public Academic Hospital, Surabaya, Indonesia
3 Department of Internal Medicine, Faculty of of Medicine, Universitas Airlangga – Dr. Soetomo Public Academic Hospital, Surabaya, Indonesia
Abstract
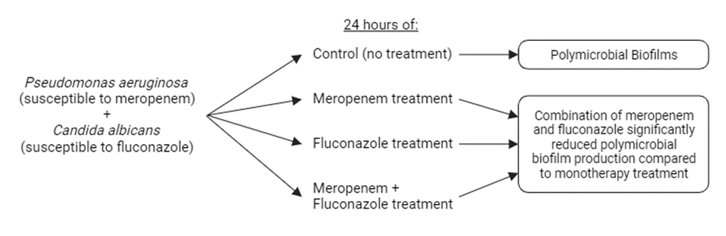
Polymicrobial biofilms, consisting of Pseudomonas aeruginosa and Candida albicans, pose a significant challenge in the field of microbiology due to their antimicrobial resistance. This study aims to investigate the potential effects of combined therapy involving meropenem and fluconazole on polymicrobial biofilms formed by these two species. Employing a true experimental laboratory design with a post-test-only control group, 32 stored clinical isolates, including meropenem-susceptible Pseudomonas aeruginosa and fluconazole-susceptible Candida albicans, were randomly selected. Polymicrobial biofilms of Pseudomonas aeruginosa and Candida albicans were established using a microtiter plate biofilm assay. After 24-hour exposure to meropenem, fluconazole, or a combination of meropenem and fluconazole, the biofilms formed were stained with 0.1% crystal violet. Optical density (OD) measurements were obtained using a spectrophotometer (ELISA reader). Data analysis using parametric ANOVA revealed significant differences (p < 0.05) in the statistical test results. Subsequent Post Hoc Test Least Significant Difference (LSD) analysis demonstrated no significant differences (p > 0.05) in the group treated with monotherapy of meropenem and fluconazole, while a significant difference (p < 0.05) was observed in the group treated with the combination therapy. The decline in optical density observed in this study could be attributed to a reduction in the extracellular matrix of the biofilm, a decline in the number of viable microbial cells, which subsequently reduces the production of the biofilm matrix, or a combination of both factors.
Graphical Abstract
Keywords
Main Subjects
Introduction
The majority of infections in humans manifest as polymicrobial, challenging the traditional understanding of diseases caused by a single etiologic agent [1]. Polymicrobial infections entail the presence of two or more species of microorganisms, regardless of titer level or infection location. Common polymicrobial infections include periodontitis, gastroenteritis, diabetic foot ulcers, burns, and biofilm-related infections. This phenomenon can manifest in various human body organs, encompassing both external and internal organs [2].
The complexities of polymicrobial infections make them challenging to treat, primarily due to a lack of comprehensive understanding regarding how pathogens interact during infections and how these interactions influence the efficacy of drugs and can lead to a worsened disease prognosis. For instance, in wound care, polymicrobial infections can impede the healing process and compromise the integrity of soft tissue [3,4]. Moreover, microbial interactions can augment the production of the extracellular polymeric substance (EPS) matrix constituting biofilms. Biofilm formation can be initiated by either bacteria or fungi and can potentially accelerate microbial growth and induce antimicrobial drug resistance [5]. Notably, one of the polymicrobial interactions associated with biofilm formation is the coinfection involving Pseudomonas aeruginosa and Candida albicans [1,6].
Coinfections of fungi and bacteria contribute to escalated mortality rates. In burn patients, candidemia frequently exacerbates due to coinfection with Gram-negative bacteria, particularly Pseudomonas aeruginosa [7]. Likewise, the colonization of Candida spp. in the respiratory tract heightens the risk of ventilator-associated pneumonia (VAP) caused by Gram-negative pathogens [8].
Pseudomonas aeruginosa and Candida albicans represent two of the most prevalent opportunistic pathogens in developed countries, occupying analogous niches and being linked to polymicrobial infections. Candida albicans ranks as the fourth most common nosocomial pathogen, while Pseudomonas aeruginosa is a significant monomicrobial pathogen [9]. Coinfections involving Pseudomonas aeruginosa and Candida albicans exacerbate diseases, but the appropriateness of treating coinfections with the same antimicrobials as single infections remains unclear [4].
Meropenem stands as the primary antibiotic for treating Pseudomonas in cystic fibrosis (CF) lung infections. This β-lactam carbapenem targets penicillin-binding proteins (PBPs) in Gram-negative bacteria, inhibiting cell wall peptidoglycan synthesis, ultimately inducing osmotic lysis of the bacterial cell [1]. Theoretically, meropenem administration is effective against Gram-negative bacterial infections like Pseudomonas spp. [10]. However, in the context of polymicrobial infections involving Pseudomonas aeruginosa and Candida albicans, the resulting biofilm can impede drug penetration, fostering antibiotic tolerance to meropenem, cefepime, piperacillin-tazobactam, ciprofloxacin, and levofloxacin. The incidence of meropenem resistance varies between 20.1% to 38.3% [11].
Research conducted at the Institute of Medical Science, India, discovered that 29.1% of Pseudomonas aeruginosa isolates were biofilm producers, with planktonic sensitivity to antibiotics like meropenem at 78.5%, piperacillin-tazobactam at 74.6%, levofloxacin at 67.2%, amikacin at 65.7%, and ceftazidime at 35.8% [12].
Fluconazole, a first-generation triazole inhibitor, is an antifungal drug exhibiting fungistatic activity against Candida albicans, Candida tropicalis, and Candida glabrata. This drug is classified within the first-line antifungal group and has been proven effective in treating fungal infections such as candidiasis [13], dermatophytosis, and aspergillosis. It offers the most favorable benefit-risk ratio for patients, ensuring quality, stability, bioavailability, and a high benefit-cost ratio based on both direct and indirect costs. It is easily accessible and well-established [14,15]. Research in India reported fluconazole's effectiveness at approximately 87.8% for Candida albicans and around 68.9% for non-albicans species. Notably, Candida albicans may develop resistance to fluconazole, especially during prolonged therapy [16,17].
A study by Hattab et al. indicated that Pseudomonas aeruginosa can enhance the activity of fluconazole, a fungistatic antifungal drug, in vitro [4].
Coinfection with Pseudomonas aeruginosa and Candida albicans potentially elevates the risk of meropenem tolerance. Extracellular matrix substances produced by Candida albicans may diminish meropenem's effectiveness, although the precise mechanism remains uncertain [1]. The amalgamation of meropenem and antifungals like fluconazole could represent an effective therapeutic alternative in cases of polymicrobial infections [4]. This research was conducted to assess the efficacy of combination therapy involving meropenem and fluconazole against polymicrobial biofilms (Pseudomonas aeruginosa and Candida albicans) in vitro, measured through optical density (OD) values. The outcomes of this study are expected to contribute to the scientific evidence supporting the effectiveness of combined meropenem and fluconazole therapy in managing polymicrobial infections associated with biofilms.
Materials and methods
Sample selection
A total of 32 clinical isolates, consisting of Pseudomonas aeruginosa and Candida albicans, were retrieved from the Clinical Microbiology Unit at Dr. Soetomo Hospital, Surabaya. The selection process involved random sampling, adhering to specific inclusion criteria: (1) Pseudomonas aeruginosa identified using the BD PhoenixTM semi-automatic system and Candida albicans identified using the Vitek® 2 Compact system, (2) Pseudomonas aeruginosa susceptible for meropenem identified using the BD PhoenixTM semi-automatic system, (3) Candida albicans susceptible for fluconazole using the Vitek® 2 Compact system, and (4) Pseudomonas aeruginosa and Candida albicans producing biofilms.
Research design
This study encompassed three treatment groups: (1) Pseudomonas aeruginosa and Candida albicans exposed to meropenem (5 mg/ml) [1], (2) Pseudomonas aeruginosa and Candida albicans exposed to fluconazole (2.5 mg/ml) [18], and (3) Pseudomonas aeruginosa and Candida albicans exposed to a combination of meropenem (5 mg/ml) and fluconazole (2.5 mg/ml). Subsequently, Pseudomonas aeruginosa and Candida albicans, capable of producing biofilms, were subcultured on MacConkey and Sabouraud Dextrose Agar (SDA) media for 24 hours at 37 °C. Following this, 3-5 colonies from each clinical isolate were cultured for an additional 24 hours. A suspension of 0.5 McFarland (1.5 x 108 CFU/ml) was prepared in normal saline (NS), supplemented with 5% glucose. This suspension was then introduced into a microtiter plate by filling the plate with 100 μL Tryptic Soy Broth (TSB) + 50 μL Pseudomonas aeruginosa suspension + 50 μL Candida albicans suspension + 20 μL 5% glucose, followed by incubation for 24 hours (biofilm formation phase). After the biofilm formation process, a biofilm eradication test was performed. The media in the microplate biofilm assay were replaced with 100 μl new TSB + 50 μL meropenem solution + 50 μl fluconazole solution and incubated for an additional 24 hours. Following the incubation period, the microplate was washed with PBS (3x), fixed with methanol, and stained with 0.1% crystal violet and ethanol. Test results were determined using a spectrophotometer (ELISA reader) and expressed as optical density (OD).
Data analysis
Statistical analysis of the research data was conducted using the ANOVA method to assess for significant differences. The data analysis was performed employing SPSS software, and graphical representations were generated using GraphPad Prism version 8.
Results
ELISA measurements of the biofilm produced by each isolate are presented in Table 1 and Figure 1.
In Table 2, it is evident that there were differences in the mean eradication of biofilm with meropenem (49.52%), fluconazole (45.92%), and meropenem + fluconazole (71.85%) in vitro (Figure 2).
The eradication of the biofilm in this study was calculated using the formula:
% Eradication = (Mean OD control – Mean OD treatment)/Mean OD control X 100%
Mean OD control
A normality test was conducted using Shapiro-Wilk, indicating that the data followed a normal distribution (p > 0.05), as depicted in Table 3. Subsequently, a parametric ANOVA test was performed. The statistical analysis revealed significant differences in optical density with a p-value of 0.001 (p < 0.05).
Next, a Post Hoc Test Least Significant Difference (LSD) was conducted (Table 4), which demonstrated significant disparities in biofilm eradication for single therapies of meropenem and fluconazole as well as combination therapy of meropenem + fluconazole (p < 0.05) compared to the positive control. However, no significant difference was observed between single therapies of meropenem and fluconazole (p > 0.05). Notably, there was a significant difference in biofilm eradication for combination therapy of meropenem + fluconazole (p < 0.05).
Discussion
Polymicrobial biofilms, particularly those composed of Pseudomonas aeruginosa and Candida albicans, pose a significant challenge in microbiology owing to their resistance to antimicrobial agents [19]. The primary objective of this study is to investigate the potential of combination therapy involving meropenem and fluconazole in inhibiting the formation of polymicrobial biofilms by these two species. A deeper understanding of this interaction could provide critical insights for the development of novel and effective treatment strategies against polymicrobial biofilms.
The findings of this research demonstrated a reduction in optical density within polymicrobial biofilms (Pseudomonas aeruginosa and Candida albicans) upon exposure to the combination of meropenem and fluconazole compared to the control group. In addition, a decline in optical density was observed compared to the treatment group receiving meropenem and fluconazole as single agents. This substantiates that the combination of meropenem and fluconazole effectively inhibits the formation of polymicrobial biofilms by Pseudomonas aeruginosa and Candida albicans in vitro.
Meropenem has demonstrated antibiofilm properties against various Gram-negative rod bacteria in previous studies [20-22]. Haagensen et al. (2017) demonstrated that a 24-hour and 72-hour exposure to meropenem led to the rapid and sustained destruction of Pseudomonas aeruginosa strain PAO1 biofilms. Meropenem selectively eliminates subpopulations located on the biofilm surface, irrespective of the biofilm maturation level [21].
The bactericidal effect of carbapenems on biofilm-residing bacteria has been associated with the disruption of biofilm architecture in Haemophilus influenzae [23] and Klebsiella pneumoniae [24]. However, limited studies have elucidated how bacteria eradication impacts the architecture of established biofilms. The extracellular matrix within biofilms comprises a blend of extracellular DNA (eDNA), lipids, polysaccharides, and extracellular proteins, providing structural integrity and mechanical stability to the adherent bacterial population. Several proteins in the extracellular matrix organize into structures that attach to bacterial cells through specific proteins. The cells elimination from the biofilm's outermost layer can disrupt the interaction between bacterial cells and attachment proteins in the biofilm, consequently damaging the biofilm architecture [20].
Extracellular DNA (eDNA) indeed plays a crucial role in biofilm formation and stability by providing mechanical support. Furthermore, eDNA within the biofilm matrix increases resistance to cationic antimicrobial peptides and aminoglycosides, although it does not impact resistance to beta-lactams [25]. This characteristic likely contributes to the antibiofilm activity of carbapenems. Imipenem, for instance, has demonstrated significant antibiofilm effects by reducing eDNA levels [20]. The absence of eDNA can impede biofilm formation and disrupt biofilm architecture [26].
The research outcomes highlighted that in addition to its anti-biofilm effects against Gram-negative rod bacteria, meropenem inhibited the growth of Candida spp. in both planktonic and biofilm forms. Meropenem significantly reduced the cellular activity of Candida spp. biofilms, affecting both developing and mature biofilms [27].
However, fluconazole, when utilized as a monotherapy, displayed limited antibiofilm activity when exposed to Candida albicans biofilms cultured under dynamic culture conditions (flow conditions), primarily affecting cell dispersion from the biofilm [28]. The resistance of Candida albicans biofilms to fluconazole can be attributed to a specific transcriptional response of sessile Candida albicans cells, leading to increased expression of genes involved in ergosterol biosynthesis and the efflux pump [29].
In a study conducted in 2022, fluconazole demonstrated antibiofilm activity against Candida albicans cultured on dental prosthesis support materials. As fluconazole concentrations increased, it reduced the metabolic activity and viability of Candida albicans cells in biofilms. However, complete inhibition was not achieved even at the highest concentration tested [29].
The observed decline in optical density in this study when polymicrobial Pseudomonas aeruginosa and Candida albicans biofilms were exposed to a combination of meropenem and fluconazole could be attributed to several factors: (1) a reduction in the biofilm's extracellular matrix due to declined production or structural degradation, (2) a decline in the number of viable microbial cells, thus reducing biofilm matrix production, or (3) a combination of both factors.
Several limitations are acknowledged in this research. The study utilized optical density as a measuring parameter, representing the biofilm's biomass, without providing insights into microbial viability within the biofilm or the biofilm structure. In addition, the research was conducted under static culture conditions, which may not accurately mimic the clinical conditions associated with polymicrobial Pseudomonas aeruginosa and Candida albicans infections.
Conclusion
In conclusion, the combined therapy involving meropenem and fluconazole demonstrates notable efficacy in reducing the production of polymicrobial biofilms formed by Pseudomonas aeruginosa and Candida albicans. These findings provide a strong rationale for considering the meropenem administration in conjunction with fluconazole when encountering clinical infections confirmed to be caused by a polymicrobial biofilm of Pseudomonas aeruginosa and Candida albicans bacteria.
Acknowledgements
The author would like to thank all the hospital staff of Dr. Soetomo Surabaya for helping us with this research
Conflict of Interest
All author declare no conflict of interest.
Orcid:
Budi Mulyawan: https://orcid.org/0009-0004-4409-7278
Agung Dwi Wahyu Widodo: https://orcid.org/0000-0002-3449-768X
Muhammad Vitanata Arfijanto: https://orcid.org/0000-0003-4510-755X
--------------------------------------------------------------------------------------------
How to cite this article: Budi Mulyawan, Agung Dwi Wahyu Widodo*, Muhammad Vitanata Arfijanto. Effect of meropenem and fluconazole combination therapy on polymicrobial biofilms (Pseudomonas aeruginosa and candida albicans): an in vitro study. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(1), 21-31. Link: http://jmpcr.samipubco.com/article_182753.html
--------------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
