Author
1 Department of Medical Biology and Biochemistry, Faculty of Medicine Universitas Diponegoro, Semarang Indonesia Semarang-50275, Central Java, Indonesia
2 Department of Pediatrics, Diponegoro National Hospital, Semarang-50275, Central Java, Indonesia
3 Division of Pediatrics, Williambooth General Hospital, Semarang-50131, Central Java, Indonesia
Abstract
Nephrotic syndrome (NS) is defined as severe proteinuria that results in low albumin levels, increased permeability within the glomerular filtration barrier, and functional impairment. Oxidative stress and inflammation are believed to play a significant role in the development of nephropathy. Reactive oxygen species, mitochondria, nitric oxide (NO) synthases, and xanthine oxidases are all injured by kidney-inducing substances. This study aimed to elucidate the mechanism of hypoalbuminaemia in nephrotic syndrome related to oxidative stress in NS. Through studies and reviews, the causes, pathophysiology, sources, and agents of renal oxidative stress have been elucidated over several decades. We reviewed studies on reactive oxygen species (ROS) formation and their relationship with hypoalbuminaemia nephrotic syndrome. The pathogenic pathways that lead to renal fibrosis, mechanisms of oxidative stress production during renal disorders, and medications that specifically target oxidative stress during tubulointerstitial fibrosis and glomerulosclerosis are explained in this article. A distinguishing feature of NS is increased excretion of albumin and other serum proteins. Therapies that target oxidative stress have the potential to treat renal fibrosis, given the importance of oxidative stress in renal nephrotic syndrome.
Graphical Abstract
Keywords
Introduction
Nephrotic syndrome (NS) is defined as severe proteinuria that results in low albumin levels, increased permeability within the glomerular filtration barrier, and functional impairment. Although glucocorticoids are the cornerstone of NS processing, they cause significant side effects and resistance in many patients, thereby increasing the risk of chronic or end-stage renal impairment. The aetiology of NS is complex and includes immunological dysfunction, oxidative stress, and molecular changes in the podocytes. Free radicals in the human body are known oxidants. Oxidants in the human body include endogenous and/or exogenous oxidants. The human body functions as an oxidant. These ants include superoxide anions (O2), reactive oxygen species (ROS), hydroxyl radicals (OH −), singlet oxygen (1O2), and hydrogen peroxide (H2O2) [1]. Oxidants in the human body include endogenous and/or exogenous oxidants [2].
Free radicals and other ROS can be produced by both internal and external factors such as exposure to the use of X the ozone layer, cigarette smoke, air pollution, and industrial toxic substances. Free radicals are continuously produced within cells via enzymatic and nonenzymatic activities. Several enzymatic processes can produce free radicals, including the cytochrome P-450 framework, prostaglandin phagocytosis, and respiratory chains [3]. Prooxidants can also be produced by ionising or nonenzymatic interactions between oxygen and organic molecules. All molecules of oxygen (O2) are included in the word "prooxidants”, and they have a higher reactivity than superoksida, hydroxyl (HO-), and hydrogen peroxide (H2O2). ROS is produced in the tubules of the body via normal aerobic metabolism. Oxidants' nature was somewhat more stable than that of non-oxidant compounds, but with significantly higher levels of activity [4]. This review article tries to figure out how oxidative stress is linked to hypoalbuminemic nephropathy and the progress made in making antioxidants that can lessen the damage to podocytes that happens as a result. The endothelium, podocytes, and foot processes line glomerular capillaries, forming a glomerular filter. The larger proteins were eliminated after filtering. Podocyte damage can result in irreversible damage to the glomerulus. Proteinuria can occur because of injury to the basement membrane of the glomerular system, epithelial surface, or podocytes. Albumin, the major component, accounts for 85% of proteinuria cases. Nonselective proteinuria induced by glomerular permeability abnormalities could not be distinguished. Therefore, this review aimed to determine how oxidative stress affects hypoalbuminaemia and nephropathy related to nephrotic syndrome.
Nephrotic syndrome, clinical spectrum, and laboratory hallmarks
Children aged > 1 year with NS (>80%) and steroid-sensitive nephrotic syndrome (NSSS) in remission after steroid therapy often show full enhancement in accordance with symptoms. However, steroid-resistant nephrotic syndrome (NSRS) is found in 20% of children older than one year, and the majority of infants are younger than one year. However, some children with steroid-sensitive nephrotic syndrome (NSSS) experience remission [5,6].
Many proteins in the urine (40 mg/m2 LPB/h, urine protein/creatinine ratio > 2 mg/mg, or dipstick 2+) are signs of nephrotic syndrome (NS), a medical condition. Other symptoms include oedema, hypoalbuminemia (2.5 g/dL), and the potential presence of hypercholesterolaemia [7]. High homocysteine levels and insufficient vitamin B levels can lead to thrombosis. NS is a condition that requires strict parental home oversight, adjustment of medication dosages, and daily intake protocols, which can lead to relapse and increased complexity. Our previous study indicated that the intervention group had somewhat higher average medication adherence compliance [8]. Children who lose protein in their urine may develop life-threatening consequences such as infection and thrombosis. Within the first three months of life, congenital nephrotic syndrome develops, infantile nephrotic syndrome develops between four and 12 months of life, and paediatric nephrotic syndrome develops beyond one year of life [9]. The clinical spectrum of NS is shown in Figure 1.
Childhood NS is divided into three categories: congenital (less than 1%), secondary (10%), and idiopathic (INS; 90% of cases). The two primary histological forms of idiopathic NS were MCD (85%) and FSGS (Focal Segmental Glomerulosclerosis (FSGS) (10%). Systemic conditions, including Alport syndrome, Henoch-Schonlein purpura nephritis, and lupus nephritis, are considered secondary NS. Severe proteinuria before the age of three months is referred to as congenital NS and is associated with CMV, syphilis, or other congenital infections as well as abnormalities in the genes that code for podocyte proteins (NPHS1: NPHS1: Congenital Nephrotic Syndrome of the Finnish type, NPHS2: NPHS2 Stomatin Family Member, NPHS2: NPHS2 Stomatin Family Member, and WT1: Wilms tumour protein gene) [5,10].
Hypoalbuminemia in Nephrotic syndrome
Increased glomerular capillary wall permeability, which results in severe proteinuria and hypoalbuminaemia, is the primary anomaly observed in NS patients. Because of the extreme flattening of podocyte foot processes (a characteristic of idiopathic nephrotic syndrome) observed in patients with NS, podocytes may be significant in the biopsies of these patients [10,11]. Complex immune system diseases, particularly T cell-mediated immunity, have been linked to idiopathic nephrotic syndrome. A subgroup of activated lymphocytes creates plasma factors that increase capillary wall permeability in focal segmental glomerulosclerosis (FSGS). Podocyte protein (podocin and -actinin 4) and podocyte gene MYH9 mutations are also linked to focal segmental glomerulosclerosis (FSGS) [12].
In nephrotic syndrome, the complex interaction between free fatty acids (FFAs) and albumin changes the molecular link between proteinuria and high triglyceride levels. FFAs are mostly used as fuel by skeletal muscles and the heart. These organs take in both albumin-bound FFAs from food and lipolysis, and FFAs are released by lipoprotein lipase (LPL)-mediated hydrolysis. When low-FFA-content albumin is lost through urine, it is replaced by high-FFA-content albumin, which changes the ratio of FFA uptake. This causes the localised overexpression of Angptl4, which inactivates LPL in tissues and reduces FFA production and hypertriglyceridaemia. The rapid absorption of albumin-bound FFA in peripheral organs and the disproportionate retention of albumin at high FFA concentrations are required for Angptl4's antiproteinuric and hypertriglyceridemia-inducing actions. The NPHS2 (podocin) and WT1 genes, as well as other parts of the glomerular filtration system, such as the slit pore containing nephrin, NEPH1, and CD-2-related proteins, can be mutated in steroid-resistant nephrotic syndrome. Hypoalbuminemia Hypoalbuminemia is the systemic condition that proteinuria is most closely associated with. If the albumin level is less than 2.5 g/dL, hypoalbuminaemia is one of the signs and symptoms of nephrotic syndrome in children [13]. The liver usually produces 12-14 g of albumin per day (130-200 mg/kg), which is metabolised by the same metabolite. After filtered albumin is absorbed, 10% is catabolised in the proximal tubule of the kidney, where it occurs extrarenally [14]. Hypoalbuminaemia is a symptom of enhanced albumin catabolism and excessive urine protein loss in patients with nephrotic syndrome. Albumin loss through urine is a major factor in the occurrence of hypoalbuminaemia [15]. This is not the only cause of albumin loss through urination in individuals with nephrotic syndrome, although the rate of albumin production can increase by at least twofold. Hypoalbuminaemia is also assumed to be influenced by increased albumin loss in the gastrointestinal tract; however, there is scant evidence to support this claim [16]. Therefore, an adequate link between the decline in the rate of albumin synthesis in the liver and the subsequent increase in albumin catabolism is required for the development of hypoalbuminaemia. While studies on patients with hypoalbuminaemia related to nephrotic syndrome have demonstrated that the rate of albumin production in the liver is only slightly above normal, under normal circumstances, the rate of albumin synthesis in the liver may increase up to 300% [17], indicating that the liver does not respond adequately to albumin synthetically. The plasma oncotic pressure, which perfuses the liver, is a key regulator of protein production. A two-fold increase in the rate of hepatic albumin gene transcription compared with normal is experimental data suggesting a hereditary shortage of circulating albumin. However, the increase in albumin synthesis in the liver was insufficient to offset the degree of hypoalbuminaemia, indicating a compromised synthetic response [18].
This also occurs in patients with NS; a drop in oncotic pressure is inadequate to speed up liver albumin production enough to increase plasma albumin concentration. In addition, there is evidence that albumin synthesis is controlled by the hepatic interstitial albumin levels in healthy individuals. The response to albumin production is normal and increases slightly but insufficiently in nephrotic syndrome because the hepatic interstitial albumin pool is not depleted. Albumin production is affected by dietary protein consumption [19] and an increase in hepatic protein and albumin mRNA synthesis, whereas a low-protein diet does not [20]. Hyperfiltration caused by increased protein ingestion also results in increased albuminuria, whereas the serum albumin levels are unaffected. There is an ongoing debate regarding whether renal albumin catabolism causes hypoalbuminaemia in nephrotic syndrome. An increase in filtered protein that is only released in the urine, rather than being taken in and digested, indicates that the ability of renal tubules to transport albumin has reached its saturation threshold at healthy levels of processed albumin [21]. Studies on the perfusion of isolated proximal tubules in rabbits have shown that albumin absorption uses a dual transport system [22]. In this study, there are two types of structures: a low-capacity system that stops working when there is too much protein, and a high-capacity system with low affinity that allows the tubular rate of albumin absorption to increase as the filtered load increases. This theory is reinforced by the positive relationship between albumin fraction catabolism and albuminuria caused by puromycin aminonucleoside PAN (Puromycin Aminonucleoside-Induced Nephrosis) [23,24]. However, the absolute catabolic rate may be normal or even lower in nephrotic syndrome because of significantly decreased total body albumin storage. The fact that absolute albumin catabolism is decreased in nephrotics on a low-protein diet, but not when they consume a regular amount of protein, is proof that this has an impact on their nutritional status [25]. It is now obvious that numerous modifications in albumin homeostasis that cause low albumin levels in nephrotic syndrome can cannot be completely compensated for by decreasing tubular renal albumin catabolism and boosting hepatic albumin synthesis. Several theories have been proposed to explain the occurrence of oedema in nephrotic individuals. The subjects of this study were the traditional hypothesis regarding oedema formation [26]. Accordingly, fluid seeps into the interstitial space because of oedema caused by a drop in the intravascular oncotic pressure. Albumin leakage and hypoalbuminaemia are caused by an increase in glomerular capillary permeability. The determination of oncotic pressure is an essential activity of albumin. Therefore, the intravascular plasma colloid oncotic pressure decreases owing to hypoalbuminaemia. Oedema occurs when transudate fluid from the intravascular area leaks past the capillary walls into the interstitial space [17]. Figure 2 depicts the pathophysiology and mechanism of oedema development in patients with NS. According to the overflow hypothesis, 16 primary intrarenal processes regulate renal salt and water retention, as opposed to peripheral systemic stimulation. Primary renal salt retention causes a spike in the amount of extracellular fluid and the plasma. When there is too much fluid in the interstitial space, oedema develops [27]. Hypercholesterolaemia-nephropathy syndrome is characterised by an increase in almost all lipid levels (cholesterol, triglycerides, and serum lipoproteins). There are several possible explanations for this, including hypoproteinaemia, which increases the synthesis of all proteins in the liver, including lipoproteins [28]. A decrease in plasma levels of lipoprotein lipase, the primary enzyme system that removes fat from the plasma, also contributes to a decrease in fat catabolism. Hypoalbuminaemia, hyperlipidaemia, and peripheral oedema are caused by reduced glomerular capillary wall permeability, which also results in the failure to maintain protein loss in the urine [29].
Several clinical disorders have been associated with free radical oxidative damage, in kidney injury [30,31].
Stress oxidative role in Nephrotic syndrome
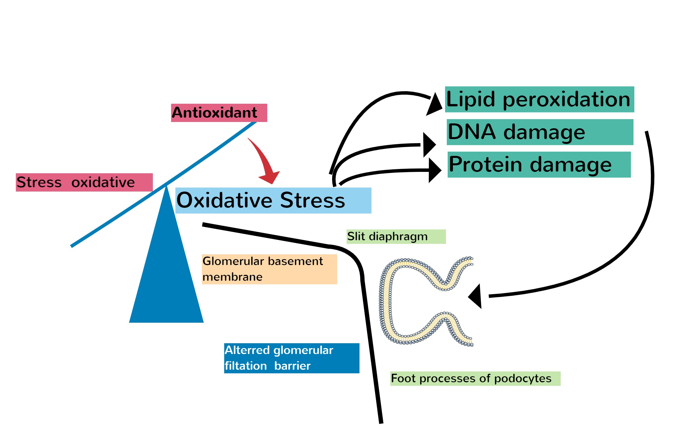
Glomerular illnesses with diverse aetiologies are characterised by albuminuria. It is a cause of glomerular disease and is not only one of its symptoms, but also triggers interstitial fibrosis, glomerulosclerosis, and eventually a loss in kidney function. Reactive oxygen species (ROS) affect glomerular haemodynamics, decrease tubular epithelial cell structural integrity, and increase glomerular responsiveness to proteins, all of which contribute to cell damage via lipid peroxidation. Antioxidants which protect podocytes from ROS-induced cell death can be used to treat podocytopathy, as this system is a major factor in the emergence of podocytopathies. As free radicals are oxidative stressors in a number of disorders, their impact on the cause of nephrotic syndrome (NS) is significant [32]. The basis of this study was oxidative damage caused by free radicals, which has been associated with an assortment of clinical illnesses, including kidney damage [33]. Figure 3 shows the interaction between oxidative stress and podocyte-related injury. Children with N.S. are more likely to experience recurrence because they are more susceptible to repeated infections. Therefore, an individual's vulnerability to sickness may be influenced by reductions in antioxidants, including superoxide dismutase (SOD), glutathione (GSH), plasma (ceceruloplasmin (, and antioxidant vitamin E. These antioxidants also aid the immune system by reducing harm and adjusting to challenging circumstances [34,35]. Idiopathic nephrotic syndrome (NSI) is a T-cell disorder in which cells release cytokines that affect the glomeruli and improve plasma protein permeability. A particular type of T cell that aids in maintenance is known as a regulatory T cells [36]. Several illnesses are managed with T-cell function inhibitors such as cyclosporine and corticosteroids. Immune system failure can cause selective proteinuria, which reduces the amount of circulating substances that modify the diaphragm gap [37].
Focal segmental glomerulosclerosis (FSGS) and minimal change disease are two major symptoms of NS. MCD is the most common cause of NS among children. The exact cause of NS remains unknown [38]. However, based on the response of NS to immunosuppressive drugs such as corticosteroids and calcineurin inhibitors, which modulate T cell function, NS is believed to result from primary T cell failure [39].
A redox protein called CHOP-TXNIP (C/EBP homologous protein)-TXNIP (thioredoxin-interacting protein) is crucial for both mitochondrial dyshomeostasis and ER failure, the study's findings indicate. NS triggers mitochondrial oxidative stress (ROS). Through triggering TXNIP translocation from the nucleus to the mitochondria, CHOP up-regulates Trx2 and frees ASK1. CHOP removal inhibits TXNIP translocation and ROS overproduction, suggesting a possible therapeutic strategy for NS [40]. Table 1 summarises nephrotic syndrome supplementation that may be therapeutically useful for improving nephropathy and its proposed mechanism.
Many studies have attempted to determine how NS regulation affects the expression, operation, and release of cytokines on T-cell surfaces. Several other networks have been suggested to be connected to the NS. TNF, CLC-1, and IL-18 are the three cytokines that cause tissue damage [49]. The expression of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b), angiopoietin-like 4 (Angptl4), and glomerular podocyte B7-1 (CD80) has been observed to be impaired by these molecules, regardless of whether the individual is in a state of remission or relapse. The present theoretical framework pertaining to podocyte abnormalities and their influence on glomerular filtration in the pathogenesis of proteinuria offers an explanation for the disruption of the filtration barrier [50]. The molecules mentioned, namely sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b), angiopoietin-like 4 (Angptl4), and glomerular podocyte B7-1 (CD80), have been found to interfere with the expression of these molecules. This deficiency occurs independently of an individual's status, whether in remission or relapse [51]. In accordance with previous studies, people who have nephrotic syndrome release more inflammatory cytokines, such as IL-1, IL-2, and TNF. In MCD-related nephropathy, elevated IL-13 levels are associated with proteinuria, high cholesterol levels, and podocyte fusion in feet. Immunosuppressive drugs reduce the symptoms of nephrotic syndrome, which is functionally caused by inflammatory cytokines [52].
Despite previous reports of lower SOD concentrations, this study discovered that cellular GSH and plasma levels of vitamin E decreased in patients with nephrotic syndrome. Although ceruloplasmin (CP) is a strong antioxidant and free radical scavenger, plasma CP levels increase. However, unlike earlier studies that found an increase in MDA levels in nephrotic syndrome, erythrocyte MDA, a byproduct of the lipid peroxidation process, failed to show any appreciable alterations [53].
Research has shown that antioxidant levels are important for the prevention nephrotic syndrome. Homocysteine causes oxidative changes to LDLC and NS and affects how well steroids work [54,55].
Podocytes, a type of developing cell in the kidney basement membrane, prevent proteins from escaping in the urine by making processes which prevent protein loss. The interdiaphragm obstructs the kidneys, allowing certain substances to pass through it. FP podocytes contract and move and are held in GBM by 3/1-integrin proteins and dystroglycans. NS family gene studies have identified SD proteins, such as podocin, nephrin, -actinin-4, and TRPC6, which demonstrate how SD is placed in tandem [56].
MCD is characterised by selective albuminuria associated with FP depletion. No mutations were detected in any of these patients. This is because NS nephrins are unlikely to be produced by SD. The albumin receptor transport system moves albumin through podocytes via endocytosis and exocytosis, which explains selective albuminuria through MCS [57].
Antioxidants in nephrotic syndrome
For thousands of years, herbal medicines have been used as alternative treatments for a wide spectrum of illnesses. The antioxidative qualities of herbs and their components that come from them have been investigated for their possible therapeutic use in podocytopathies. Table 2 summarises herbs and components used for antioxidant supplementation in cases of idiopathic nephrotic syndrome, animal experimentation, and possible targeted mechanisms. These herbs and their components reduce intracellular reactive oxygen species (ROS) levels by upregulating the respiration of mitochondrial chain complex activities and downregulating pro-oxidative enzymes. In addition, they control the ROS-mediated apoptotic pathways. Geniposide, an ingredient of Gardenia jasminoides Ellis, can mitigate podocyte damage and inhibit nephropathy formation [58].
Abnormalities in Cu and Zn metabolism have been documented in NS patients. Elevated homocysteine levels and low levels of B-vitamins involved in metabolism play a role in thrombosis [59]. Significant increases in serum lipid peroxidation levels of homocysteine and decreases in serum antioxidant capacity and Cu, Zn, and plasma vitamin C levels were observed in patients with NS. Antioxidant administration improves the antioxidant profile, which can play a role in preventing the appearance of kidney scar tissue [60].
In patients with renal disease, deep antioxidant therapy has the potential to decrease oxidative stress, which is a related disease. Numerous complications of nephropathy and an increase in the production of inflammatory cytokines are caused by overproduction of reactive oxygen species and free radicals in the body. Magnesium and zinc, in combination with vitamins C and E, enhance glomerular but not tubular functions [61]. Following antioxidant and mineral therapies with B-complex vitamins, significant decreases in lipid peroxide homocysteine and increases in vitamin C, total antioxidant capacity, and copper and zinc activities were noted. Research has demonstrated that B complexes, vitamins, minerals, and antioxidants can prevent hyperhomocysteinemia and lipid peroxidation [62].
The proportion of children in the fortification group who were prehypertensive or hypertensive was significantly lower than that of the control group. Combining the antioxidants tocopherol and vitamin C can reduce the blood pressure in rats with high blood pressure. Vitamin C helps to regulate blood pressure and protects against oxidative stress by inhibiting nitric oxide synthase. In addition, vitamins C and E also promote arterial stiffness and endothelial-dependent vasodilation [46].
Another study indicated that either the breakdown of lipids or lipid peroxidation was significantly impacted by a combination of vitamins C and E. However, it was found that taking these vitamins simultaneously reduced ox-LDL and Vascular Cell Adhesion Molecule-1 (sVCAM)-1 levels in patients who had entered remission. Nitric oxide synthase has been shown to be affected by vitamin C, which reduces blood pressure, whereas vitamin E reduces blood vessel tightness and endothelial-dependent vasodilation. Overall, supplementation with vitamins C and E showed potential in reducing ox-LDL and sVCAM-1 levels during remission [63]. Our previous study showed that temulawak and black cumin extracts have lower lipid levels in NS rats. Nevertheless, there was no discernible change in HDL or LDL levels. Further research is required to fully comprehend their hypocholesterolemic qualities, more research is required [48].
Challenges and future development
A link exists between a decrease in organ function and a decline in kidney function, which has been linked to the breakdown of the glomerular basement membrane (GBM) and is defined by a history of proteinuria. Edema can be induced in people by proteinuria resulting from nephrotic syndrome, isolated proteinuria, or hypertension. Owing to haemodynamic factors, filtration barrier properties, and molecular characteristics, functional impairment in the nephrotic syndrome (NS) improves the susceptibility of the glomerular filtration barrier to materials. GFB may become increasingly permeable if disrupted, and the main problem in nephrotic syndrome (NS) is greater glomerular capillary wall permeability, which causes severe proteinuria and low albumin levels. This is because of podocyte kidney damage, which can be observed by the fact that the foot processes of podocytes in tissues from patients with NS are thinner. ROS damages cells through lipid peroxidation, which weakens the structural stability of tubular epithelial cells and makes it easier for proteins to leak out of the glomeruli. Antioxidants can improve antioxidant makeup, which can prevent scar tissue formation in the kidneys. The glomerular filtration barrier (GFB) is composed of three layers (glomerular capillary internal endothelium, glomerular visceral epithelial cells, and podocytes), and is a part of the kidney that is most affected by NS. Foot processes or peduncles of podocytes are an important part of kidney function. Ascites in the NS can be halved by eliminating ROS and inhibiting NF-B. This was because the changes in membrane leakage caused by protein reflection levels ceased. Further studies are warranted to identify the association between reactive oxygen species, adaptive mechanisms, and potential therapeutic strategies for reducing the burden of NS.
Conclusion
Increased sensitivity to the glomerular filtration barrier occurs with Nephrotic syndrome impairment, although the production of kidney scar tissue is prevented by antioxidants. Further investigation is required to understand the reactive oxygen species and treatment approaches.
Acknowledgements
The authors would like to thank Kusmiyati Tjahjono DK, MD, MSc, PhD, and Saphira Ayu, MD, M.Sc, for their constructive criticism of this manuscript.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Authors’ contributions
The authors fully accept responsibility for all the work's content and have given permission for it to be submitted.
Funding
No specific grant for this research was provided by funding organisations in the public, private, or non-profit sectors.
Data availability
Because the information in the review article was derived from the sources of the literature suggested in the article, it did not contain any associated data. This study was registered with the Open Science Framework (OSF).
Abbreviations
Angptl4: angiopoietin-like 4, CHOP (C/EBP homologous protein)-TXNIP (thioredoxin-interacting protein), CMV: cytomegalovirus, CP: ceruloplasmin, C0-Q10: Coenzyme Q10, ER stress-related proteins: Endoplasmic reticulum stress related protein, FFA: Free fatty acids, FSGS: Focal segmental glomerular sclerosis, GFB: glomerular filtration barrier, GSH: glutathione, HIF-1α: Hypoxia,-inducible factor-1, IL-1: Interleukin-1, IL-2: Interleukin-2, LPL: Lipoprotein lipase, LDLc; low density lipoprotein cholesterol, SRNS: steroid-resistant nephrotic syndrome, MCD: minimal change disease, NO: nitric oxide, NPHS1: Congenital Nephrotic Syndrome of the Finnish type, NPHS2: NPHS2 Stomatin Family Member, NS: Nephrotic syndrome, ox-LDL: oxidized LDL, PAN: Puromycin Aminonucleoside-Induced ROS: reactive oxygen species, RNS: reactive nitrogen species, SOD: super oxide dismutase, TNF: Tumor Necrotizing Factor, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling, VCAM-1: Vascular Cell Adhesion Molecule-1, WT1: Wilms tumor protein gene.
Orcid:
Amallia Nuggetsiana Setyawati: https://orcid.org/0000-0002-1322-1369
--------------------------------------------------------------------------------------------
How to cite this article: Amallia Nuggetsiana Setyawati, The role of oxidative stress in hypoalbubimenia nephropathy related to Nephrotic syndrome: a critical review. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(1), 32-49. Link: http://jmpcr.samipubco.com/article_182755.html
--------------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
