Document Type : Original Research Article
Authors
- Marisca Evalina Gondokesumo 1
- Faisal Akhmal Muslikh 2
- Rizki Rahmadi Pratama 3
- Burhan Maarif 4
- Dyah Aryantini 2
- Reza Alrayan 2, 5
- Dewi Luthfiana 6, 7
1 Faculty of Pharmacy, University of Surabaya, Surabaya, Indonesia
2 Faculty of Pharmacy, Bhakti Wiyata Health Sciences Institute, Kediri, Indonesia
3 Faculty of Pharmacy, Islamic University of Kalimantan Muhammad Arsyad Al Banjari Banjarmasin, South Kalimantan, Indonesia
4 Department of Pharmacy, Faculty of Medical and Health of Science, Islamic State University Maulana Malik Ibrahim, Malang, Indonesia
5 Research and Education Center for Bioinformatics, Indonesia Institute of Bioinformatics, Malang,
6 Graduate School of Bioagricultural Sciences, Department of Applied Biosciences, Nagoya University, Furo-cho, Chikusa, Nagoya, Japan
7 Research and Education Center for Bioinformatics, Indonesia Institute of Bioinformatics, Malang, Indonesia
Abstract
Neurodegenerative disorders (NDD) are age-related condition characterized by a progressive decline in brain functions. This condition has influenced more than 8% of the adult population worldwide, predominately with Alzheimer's disease (AD). Currently, NDD treatment is addressed to relieve existing symptoms, so the effective medication is urgently needed. Flavonoids offer remarkable pharmacological properties applicable to be neuroprotective agents. This study aimed to determine the activity of flavonoid compounds against AD by inhibiting acetylcholinesterase (AChE) and monoamine oxidase B (MAO-B) receptors. The method utilized molecular docking studies with the AutoDockTools 4.2.6 program. Analysis of pharmacochemical properties were carried out using SwissADME, while pharmacokinetics and toxicity were examined in the pkCSM web server. The results indicated that α-amyrin and pinoresinol were the most potential AChE and MAO-B inhibitor, respectively. The compounds have lower energy binding values, inhibition constants, and high percentage of similarity with amino acid residues in the ligand native. Analysis of the physicochemical and pharmacokinetic properties showed that these two compounds are acceptable to the body and provides no toxicity. This study demonstrated that the compounds α-amyrin and pinoresinol might potential to be therapeutic agent which primarily act to inhibit AChE and MAO-B in AD progression.
Graphical Abstract
Keywords
Introduction
Neurodegenerative disorders (NDD) are characterized by the progressive loss of neurons caused by metabolic disorders or poisoning [1]. This disease is closely related to age and is characterized by chronic, irreversible, and progressive neuronal degradation in certain areas of the brain. Many complex pathophysiological processes are involved in the development of NDD, including oxidative stress, neuroinflammation, errors in the folding and aggregation of insoluble proteins in the brain, mitochondrial dysfunction, proteolytic stress, etc. [2]. Alzheimer's disease (AD) and Parkinson's disease (PD) are the most common types of NDD, affecting more than 8% of adults aged 65 years or older worldwide [3]. In 2017, the prevalence of AD (at 16.2 million people) is higher than PD and expected to increase to 30.2 million in 2060 [4,5].
The existing treatments addressed symptomatic disease which is not effectively cure NDD [6]. Donepezil, galantamine, rivastigmine, and tacrine which act as acetylcholinesterase (AChE) inhibitors in the treatment of AD [3,7], but have side effects on the gastrointestinal, cardiovascular, and respiratory systems [8]. Monoamine oxidase B (MAO-B) inhibitor drugs are also used in AD to regulate intraneuronal Aβ levels via γ-secretase [9]. However, this drug has side effects such as dry mouth, nausea, diarrhea, constipation, drowsiness, insomnia, and dizziness [10]. Therefore, there is an urgent need to find new therapeutic agents with minimal side effects. Identification of bioactive compound derived from natural products could be a promising approach to solve the problem [11].
Flavonoids are a large class of natural aromatic compounds and reported as the most common phenolic plant. The chemical structure of flavonoids consists of a C6-C3-C6 ring, which corresponds to two aromatic rings connected by three carbon atoms, leading to the formation of a third ring [12]. This variation in basic structure gives rise to various subclasses of flavonoid compounds such as flavanones, isoflavones, flavones, flavanols (catechins), chalcones, flavonols, and anthocyanins [13-15]. This underlies different biological activities such as antioxidant, antibacterial, antihypertensive, liver protection, antitumor, anticancer, anti-inflammatory, and neuroprotective effects [12]. This study was conducted to determine the activity of flavonoid compounds against AD through an in silico study using AChE and MAO-B proteins, because these two protein targets are commonly used in AD.
Methods
Tools and materials
This study used a computer Legion 5 Pro 16AC6H specification with 16.0 GB of RAM, AMD Ryzen 7 5800H processor, NVIDIA GeForce RTX 3060 graphics Card, 3.20 GHz Radeon graphics, and Microsoft® Windows® 10 Pro. The sofware utilize Swiss PDB viewer to optimize proteins, Avogadro 1.2 for energy minimization, Discovery Studio visualizer 4.5 and PyMOL 2.5 to visualize interactions between proteins and ligands, Autodock 4.2.6 to perform molecular docking, SwissADME to predict physicochemical properties, and pkCSM to predict the pharmacokinetic properties and toxicity of the tested compounds.
Protein and ligand preparation
The AChE receptor protein was retrieved from the protein data bank web server with the PDB id. 4EY7 (https://www.rcsb.org/structure/4EY7) and the MAO-B protein with the PDB id. 2V5Z (https://www.rcsb.org/structure/2V5Z).
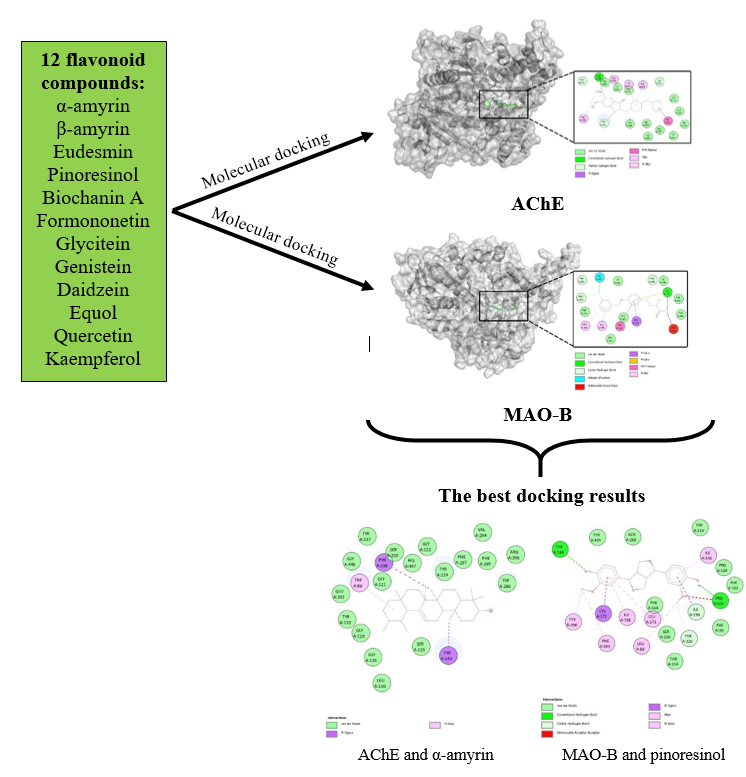
Each protein was removed from water molecule and added a hydrogen molecule using a Discovery Studio visualizer [16]. Following this, the protein structure was optimized using PDB Swiss and the force field was set using GROMOS96, then saved in ".pdb file" format. This study used 12 flavonoid compounds obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Those flavonoid compounds were familiar compounds easily found in the nature, such as α-amyrin, β-amyrin, Eudesmin, Pinoresinol, Biochanin A, Formononetin, Glycitein, Genistein, Daidzein, Equol, Quercetin, and Kaempferol [17-18]. These compounds were then minimized using the Avogadro software, with a force field setting using MMFF94 [19-20].
Molecular docking
This study used AChE receptors (PDB id. 4EY7) and MAO-B (PDB id. 2V5Z) as macromolecules and 12 flavonoid compounds as ligands. The docking simulation used Autodock 4.2.6 software. The initial stage begins with uploading proteins and ligands in the Autodock tool. Following this, torque detection and determination are performed automatically by adding Gasteiger and Kollman partial charges to the tested compounds. The grid box was setting out based on the results from the method validation. For AChE receptors, the grid box size is X = 40, Y = 40, Z = 40 with coordinates (-13.988; -43.906; 27.109), while the grid box size of MAO-B protein is X = 40, Y = 40, Z = 40 with coordinates (52.003; 156.138; and 27.950). The grid spacing was set to 0.375 Å. In this simulation, the Lamarckian Genetic Algorithm is used with a population of 150 and a maximum number of evaluations of 2,500,000 for every 100 conformations.
Docking results were evaluated by analyzing the best conformation based on the lowest energy binding score (∆G) and inhibition constant (Ki). The detected functional essential amino acid interactions were also observed by using the Discovery Studio visualizer.
Predictive physicochemical and pharmacokinetic properties (ADMET)
Analysis of pharmacokinetic and pharmacodynamic properties was carried out by converting the compound file format into a simplified molecular-input line-entry system (SMILES) using ChemDraw Ultra 12.0 software. SMILES code aimed to facilitate the analysis of pharmacokinetic and pharmacodynamic properties by comparing these compounds using IUPAC nomenclature [21-22].Furthermore, the prepared SMILES format was processed separately on the SwissADME web tool (http://www.swissadme.ch) and clicked on run to analyze the physicochemical properties of each compound. The results include molecular weight, hydrogen bond donor (HBD), hydrogen bond acceptor (HBA), log P, and TPSA values [23].
Meanwhile, the analysis of pharmacokinetic properties was carried out using pkCSM webserver (https://biosig.lab.uq.edu.au/pkcsm), the SMILES format for each compound was used to predict pharmacokinetic properties, then executing it by clicking the ADMET button.
Results and discussion
Method validation of docking simulation was carried out using Autodock 4.2.6. The result showed that the RMSD values were 1,451 and 1,899 Ǻ for AChE and MAO-B, respectively. RMSD is a benchmark used to evaluate the parameters of the docking process and describes the conformation of ligand native before and after method validation is carried out [24]. Docking is assumed valid when the RMSD value is less than 2 Å (Figure 1) [23,25].
A good indicator in the molecular docking simulation can be observed by measuring the values of the binding energy (ΔG) and the inhibition constant (Ki). The binding energy value reflects the strength of the biomolecular interaction between the ligand and the receptor [26]. The lower the energy binding value, the more stable the interaction between the ligand and the receptor, indicating a stronger affinity of the ligand for the receptor [27]. Moreover, molecular docking also produces a pose to explain the interaction of ligands with proteins [27].
This study observed that the α-amyrin compound interacted to the AChE receptor had better binding energy than its ligand native (Table 1), while pinoresinol did better than its ligand native at the MAO-B receptor. Meanwhile, the Ki value is defined as the concentration required to achieve half of the maximum inhibition. Az-Zahra et al. (2012) reported that a lower Ki value indicates that the ligand is more likely to bind to macromolecules [28]. As presented in Table 1, the Ki value of α-amyrin (2.43 nM) is better than its ligand native (6.32 nM) at the AChE receptor, and Pinoresinol (55.49 nM) is better than its ligand native (261.09 nM) at the MAO-B receptor.
The purpose of observing amino acid residues in the test compound and the target protein interaction is to identify the interactions that occur and understand the role of these interactions in the pharmacological effect of the test compound as an inhibitor of AChE and MAO-B (Figure 2). These bond interactions include hydrogen bonds, hydrophobic interactions, Van der Waals interactions, electrostatic interactions, and halogen interactions. Hydrogen bonds are the strongest of the non-covalent bonds, although they are weaker than ionic or covalent bonds. Therefore, hydrogen bonding plays an important role in enhancing pharmacological activity of the complexes [24,29].
Amino acid residues are amino acids of a protein that bind to a ligand or compound in which the active site of protein consists of different amino acids. The percentage of similarity of amino acid residues between the compound and the control (ligand native) indicates the similarity of residues in the active site, which will provide a strong bond and the same biological activity as the control [30].
Pharmacological properties of the compounds were predicted using SwissADME webserver. The results showed that flavonoids were the best compounds based on the results of molecular docking and also had properties that could be accepted by the body (Table 2). This property is evaluated based on the parameters of Lipinski's rules of five (Table 3). Several Lipinski parameters are HBD <5, HBA <10, log P <5, and molecular weight <500 g/mol [31]. Compounds with a molecular weight <500 g/mol may have the ability to pass through the biological membranes [32]. The H-acceptor and H-donor values indicate the number of hydrogen bonds in the compound. The higher values of these parameters, the higher the energy required in the absorption process. The log p-value is defined as the solubility of the compound in the membrane fluid and reflects the polarity of the compound [33]. The topological polar surface area (TPSA) value is the ability of a compound to penetrate the cell membrane of the body [34].
From the pharmacokinetic test results, it was found that α-amyrin and pinoresinol had good absorption properties as they met the criteria (can penetrate CaCO2 permeability, can be absorbed in the human intestine, and have high permeability in the skin) (Table 4). Likewise, from the distribution parameters, these two compounds can be distributed in the network because they have a range of 0.45>VDSS<-0.15 and can across the blood brain barrier (BB) and the central nervous system (CNS), suggesting that the two compounds can be targeted as drug candidates that act on the central nervous system. For metabolism parameters, indicators that can inhibit and metabolize cytochrome P450 are indicated by "Yes/No".
The two compounds were potential to be CYP3A4 substrates. While pinoresinol exhibited the ability as CYP2C19, CYPC2C9, and CYP3A4 inhibitor, those abilities were not observed in the α-amyrin. Toxicity is also the paramount parameters should be considered in designing drug candidate. The results of the toxicity test showed that the two compounds were not toxic [24,35].
The excretion analysis demonstrated that alpha-amyrin has a higher total clearance value than pinoresinol, indicating that α-amyrin has a greater excretion rate than pinoresinol. The results of these two compounds do not include Renal OCT2 substrate which shows that it does not cause toxic effects in oral preparations which are consumed together with renal OCT2 inhibitors [24,35]
MAO is a mitochondria-limited enzyme with performance levels in gastrointestinal and nervous tissue. MAO has two different isoforms including MAO-A and MAO-B. MAO causes oxidative deamination of several monoamines, so it is very important in the metabolism of several neurotransmitters that are linked to the pathophysiology of neurodegenerative diseases like Parkinson's disease, depression, and Alzheimer's disease [36]. MAO-A manifests in the gut, heart, and placenta, whereas MAO-B is limited to cerebral glial cells, platelets, and liver cells. MAO also controls mood, motor activity, and brain activity and motivation [37]. It has been observed that MAO-B in the capillaries of the BBB executes a preservative action and acts as a metabolic barricade [38]. Whereas AChE regulates cholinergic transmission at the synaptic level by hydrolyzing the neurotransmitter acetylcholine (ACh) [39]. This AChE inhibition can increase synaptic acetylcholine (ACh) levels and block the breakdown of ACh by inhibiting AChE. This AChE inhibitory effect is very important in the treatment of AD [40].
AD is characterized by the selective loss of cholinergic neurons as a consequence of reduced levels of acetylcholine (ACh) in certain brain regions that mediate memory and learning functions. Acetylcholinesterase inhibitors prevent ACh hydrolysis and thereby increase the concentration of ACh in the synaptic cleft, while MAO can enhance the neurotransmission of amines and exert valuable biochemical effects in the treatment of AD. Elevated MAO B levels due to increased astrogliosis in the brains of AD patients have been reported, suggesting that MAO inhibition may be a valuable AD therapy [41]. This dual-acting prototype inhibitor is endowed with a covalent (pseudoirreversible for AChE, irreversible for MAO) mechanism of action [39].
This study demonstrated that the α-amyrin and pinoresinol compounds have the potential to be inhibitor of AChE and MAO-B in AD. This was examined from the lower binding energy compared to the respective ligands native and has physicochemical properties that are safe when absorbed in the body. The administration of these two compounds has great potential for pharmacological activity, based on the fact that they come from natural ingredients, this pharmacological activity is due to the synergistic, antagonistic, and agonistic effects of the two compounds, thus providing a good effect in the body [42].
Conclusion
This study found that flavonoid compounds can provide neuroprotective activity on AChE receptors and MAO-B inhibitors. The best compounds in inhibiting each receptor are α-amyrin (AChE) and pinoresinol (MAO-B) because they have smaller docking energy values compared to their ligands native. The physicochemical and pharmacokinetic properties also indicate that these two compounds are acceptable to the body, suggesting its potential to be drug candidates for AD. Further research can be carried out to ensure the potency of the two compounds against each receptor corresponds to the best binding energy value, either in vitro or in vivo.
Acknowledgements
None.
Conflict of Interest
The authors declare that there is no conflicts of interest to disclose.
Orcid:
Marisca Evalina Gondokesumo: https://orcid.org/0009-0004-9774-4467
Faisal Akhmal Muslikh: https://orcid.org/0000-0002-9611-7937
Rizki Rahmadi Pratama: https://orcid.org/0000-0002-5275-7211
Burhan Ma’arif: https://orcid.org/0000-0001-9182-343X
Dyah Aryantini: https://orcid.org/0000-0002-1580-8358
Reza Alrayan: https://orcid.org/0009-0009-5277-7413
Dewi Luthfiana: https://orcid.org/0000-0001-6109-5283
-----------------------------------------------------------------------------------
How to cite this article: Marisca Evalina Gondokesumo*, Faisal Akhmal Muslikh, Rizki Rahmadi Pratama, Burhan Ma’arif, Dyah Aryantini, Reza Alrayan, Dewi Luthfiana, The potential of 12 flavonoid compounds as alzheimer's inhibitors through an in silico approach. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(1), 50-61. Link: http://jmpcr.samipubco.com/article_182761.
-----------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
