Document Type : Original Research Article
Authors
- Sri Aulia Novita 1
- Santosa Santosa 2
- Nofialdi Nofialdi 3
- Andasuryani Andasuryani 4
- Ahmad Fudholi 5
- Azril Azril 6
- Rahadian Zainul 7
1 Doctoral Student Agricultural Science Program Universitas Andalas, Padang, 25163, Indonesia, Department of Agricultural Mechanization Technology, Faculty Agricultural Technolgy, Tanjung Pati, 26271, Indonesia
2 Department of Agricultural Industrial Technology, Faculty Agricultural Technology, Padang25163, Indonesia
3 Department of Agribusiness, Faculty of Agriculture, Universitas Andalas, 25163, Indonesia
4 Department of Agricultural and Biosystems Engineering Agricultural Technology, Padang25163, Indonesia
5 Solar Energy Research Institute, University Kebangsaan Malaysia, 43600 Bangi Selangor, Malaysia, Research Centre for Electrical Power and Mechatronics, National Research and Innovation Agency (BRIN), Bandung 40135, Indonesia
6 Department of Biomedical Engineering, National Cheng Kung University, Tainan City, Indonesia
7 Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Padang, Padang, West Sumatra, Indonesia, Center for Advanced Material Processing, Artificial Intelligence and Biophysics Informatics (CAMPBIOTICS), Universitas Negeri Padang, Indonesia
Abstract
This study focuses on designing a parabolic solar pyrolysis reactor and investigating the effects of pyrolysis temperature and coconut shell particle size on the yield. It also aims to develop a mathematical model to understand the factors influencing pyrolysis and evaluate the energy aspects of solar pyrolysis. The reactor operates at temperatures between 300 °C and 650 °C, with variables like wind speed, light intensity, parabolic size, and material types affecting its performance. The process is also influenced by moisture content, reactor type, heating rate, residence time, and pyrolysis temperature. The study finds that particle size and temperature are crucial in determining the yield. It shows that specific combinations of these factors have a significant influence, with the F-calculated value being more significant at the 5% level than at the 1% level. Optimal results are observed at temperatures ranging from 500 °C to 600 °C. For instance, a 3 mm particle size at 300 °C yields 26.83%, increasing to 37.67% at 600 °C, indicating that smaller material sizes and higher temperatures enhance yields. The energy analysis reveals that the coconut shell receives between 136.29 to 454.89 W of heat, while the energy lost during pyrolysis ranges from 728.46 to 1778.976 W. The output heat energy spans from 864.75 to 2233.87 W, with energy efficiency varying from 16.35% to 30.41%. A heat balance is established using optics and thermodynamics principles, offering insights into the energy efficiency of solar pyrolysis.
Graphical Abstract
Keywords
Main Subjects
Introduction
Energy is one of the most important political issues in the world today for the ongoing fuel and economic crisis in a number of countries. It is one of the most crucial issues for any country trying to develop [1-2]. The primary and plentiful source of renewable energy is biomass, primarily composed of carbon. It comprises a blend of organic compounds combined with hydrogen, oxygen, nitrogen, and a limited quantity of other elements. Biomass primarily originates from biological sources, including agricultural leftovers (such as rice straw, wheat straw, coconut shells, empty palm fruit bunches, etc. forest remnants, municipal solid waste, and similar sources [3]. There is significant potential for the transformation of coconut shells into a renewable energy source that is capable of generating value-added products via the pyrolysis method [3-5].
Coconut shells are among the biomass residues with significant promise. Nowadays, biomass is recognized as a viable solution in an era when fossil fuel resources are diminishing and renewable sources are being increasingly embraced. By combining the convenience of traditional fuels with the renewable nature of solar energy, it is a promising option. Biomass is consistently produced from crops or waste, which makes it a viable alternative [6]. Coconut shells are a kind of biomass residue that can be transformed into a source of renewable energy in West Sumatra and Indonesia. Because coconut shells are ubiquitous, there is substantial potential for transforming them into sustainable energy sources. There are many advantages to burning coconut shells as an alternative fuel source, including their ability to disperse heat better than alternative materials such as wood and they appear as an excellent alternative fuel [7-8]. With a calorific value of 18388 kJ/kg and a carbon content of 18.29%, coconut shell has a high energy density and is easy to convert into liquid due to its high volatile content of 67.67% [9].
One approach to transforming biomass energy involves utilizing the pyrolysis process, which transforms biomass into bio-oil. Pyrolysis is a thermochemical method in which biomass waste is transformed into solid fuel (char), syngas (gas producer), and bio-oil (liquid) within a reactor, all without the presence of oxygen [10]. Pyrolysis is a thermochemical breakdown of organic matter that occurs in the absence of air or oxygen, typically within a temperature range of 300 °C to 600 °C [11-12]. The potential for advancing pyrolysis technology is substantial, given its ease of development, eco-friendly nature, and potential for economic profitability. The pyrolysis method relies on elevated temperatures for its thermochemical breakdown and thus utilizes wood or gas stoves as heat energy sources. Firewood or gas for combustion incurs substantial expenses and results in low energy efficiency [13].
In this study, concentrated solar energy is harnessed to generate heat through a parabolic collector. There is a specific parabolic shape that is employed for this project, which is known as a dish-like parabolic shape as it has been formed by applying the geometric properties of a three-dimensional parabola to the trough-like design. Direct sunlight is redirected and intensified toward a focal point receiver, enabling the system to attain operating temperatures exceeding 1000 °C. Solar energy's potency can be heightened through the adoption of concentrated solar power (CSP) technology in solar collectors. CSP is regarded as a highly promising renewable energy innovation, thanks to its capacity for generating heat and electricity, as well as its convenient means of storing thermal energy in storage devices [14-15].
CSP is a technology that collects sunlight with a collector, and then converts it into heat or electricity. A solar concentrator is a device used to collect light over a large area and focus its energy on a single focal point to increase the temperature to a higher level [16].
Utilizing thermo-solar energy and generating biofuels from biomass present potential sustainable solutions to meet the energy requirements of contemporary society while preserving the environment [17-18]. Solar pyrolysis holds potential for unlocking the energy stored in biomass through its transformation into biofuels [19]. Previous research into pyrolysis utilizing concentrated solar energy has showcased numerous advantages. However, challenges such as transient sensitivity, suboptimal heat transfer, and tar have persisted, prompting the quest for an enhanced approach to solar pyrolysis [20].
Renewable energy relies on two primary sources, namely, solar energy and biomass, which can be combined through pyrolysis to generate heat, electricity, transportation fuels, chemicals, and biomaterials [21-22]. Among various concentrated solar power (CSP) systems, the parabolic trough solar collector has received widespread development in numerous countries due to its well-established maturity and proven effectiveness [23]. Concerning this factor, the investigation in this study utilized concentrated solar energy to heat a pyrolysis reactor, using coconut shells as the main material. The chosen solar concentrator for this task was a parabolic dish equipped with a stainless steel mirror for reflecting sunlight. The objective of this study is to design a parabolic solar pyrolysis reactor, analyze the effect of pyrolysis temperature and coconut shell particle size on pyrolysis yield, design a mathematical model of factors that influence pyrolysis, and assess the energy aspects of solar pyrolysis.
Accordingly, it is hoped that some of the novelties from this research include the reactor design model and the mathematical model illustrating the influence of temperature and material size on the yield. The aim of this research is to design solar pyrolysis using a parabolic concentrator, analyze the effect of pyrolysis temperature and coconut shell particle size on pyrolysis results, and design a mathematical model of the factors that influence pyrolysis.
Materials
Coconut shell feedstock
The raw materials used in this study were coconut shells obtained from around Harau District, Limapuluh Kota Regency,West Sumatra, Indonesia. These coconut shells were dried using a dryer until the moisture content of the material was 8–11%. The dry raw materials were ground using a grinder into several sizes, namely 3 mm, 5 mm, and 10 mm. The analysis of coconut shell composition was done using proximate analysis. Proximate analysis was carried out to determine the moisture content, volatile, fixed carbon, ash content, and inorganic residue remaining after the combustion process. Theoretically, biomass with a high volatile fraction is more suitable for bio-oil production, while biomass with high fixed carbon is more suitable for biochar production [24].
Research implementation is facilitated by various equipment, encompassing workshop tools, laboratory analysis apparatus, and various measuring instruments. Among the measuring tools employed in the research are a solar power meter, an anemometer for measuring wind speed, an Infrared high-temperature thermometer, a type K thermocouple, and a stopwatch.
Concurrently, the research utilized a variety of materials, including coconut shells, recycled satellite dishes, 1 mm stainless steel plates, 0.5-inch stainless steel pipes, 1-inch stainless mirror plates, ǿ 4.5 cm iron pipes, angle iron, cutting grinding wheel, sanding grinding wheel, electrodes, 12 nut bolts, 1.5 mm stainless steel reactor tube, Arduino Uno R3, 12C LCD, power connector, and other miscellaneous materials.
Solar pyrolysis experiments
The design of the pyrolysis reactor was carried out by making functional and structural designs. The heat collector circuit was designed to produce a combustion temperature of around 300-600 °C.
The functional design described the function of each component of the pyrolysis reactor. Pyrolysis reactor components include (a) a pyrolysis tube (pyrolysis reactor), which
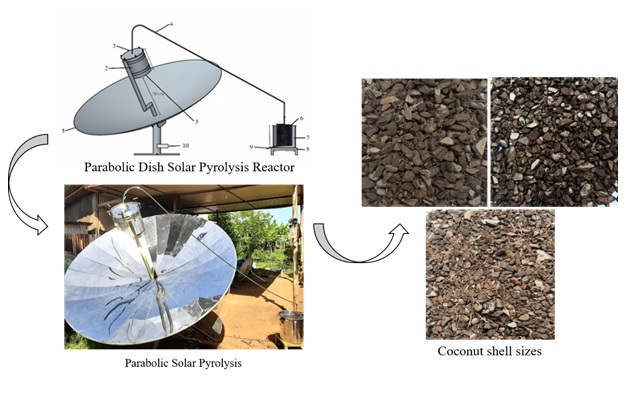
served as a place to accommodate raw materials to be burned; (b) a smoke distribution pipe, which served to drain the air or smoke produced by the combustion in the reactor; (c) Concentrated Solar Power (CSP), which served to gather solar radiation and is focused at one point to heat the pyrolysis reactor in the form of a parabolic dish; (d) the frame and stand of the concentrator, which functioned to support the position and seat of the concentrator; (e) Thermocouple type K, which was used to measure the maximum temperature in the pyrolysis reactor, (f) Arduino, which is a device that can display temperature automatically; (g) the condenser, which functions to condense hot air streams (smoke) into liquid; (h) the bio-oil discharge valve, which functions to remove bio-oil produced from the condensation process; and (i) the bio-oil reservoir, which serves to accommodate the bio-oil produced. The design of the tool can be seen in Figure 1.
The solar pyrolysis device temperature data can be obtained using an Arduino Uno. It is a circuit board with numerous components, including a USB port, a power jack, an ICSP header, and a reset button. It is important to note that Arduino is a complex and interconnected system of components that work together to make it possible for it to function as intended. Figure 2 illustrates that how the control system is developed for the pyrolysis reactor using Arduino, which allows it to determine its temperature.
The working principle of the parabolic dish concentrator
There are several working principles for parabolic dish concentrators, which include: (a) to concentrate the sunlight: the parabolic concentrator would absorb solar energy and reflect the light. The reflected light would be used to centralize the light and heat of the sun towards a focal point in the smaller area. The energy concentrated at one point would produce heat that could be used for the pyrolysis process, (b) to convert the light into heat energy, and (c) to trap the heat
Parabolic solar pyrolysis testing
The solar concentrator undergoes testing under clear sky conditions, adhering to the standard test conditions that demand a minimum solar intensity. The test standard specifies that the solar intensity value during testing must exceed 700 W/m2 [25].Various parameters are measured throughout the testing process, including solar intensity, wind speed, and ambient air temperature [26]. The instruments for measuring these parameters include a solar power meter, thermometer, anemometer, and stopwatch.
Mathematical model of the effect of temperature and particle size on yields
The form of a mathematical equation that describes multiple linear regression with two independent variables is Y = a0 + a1 X1 + a2 X2.
Where, a0, a1, and a2 are coefficients
X1 = material particle size (mm)
X2 = temperature (oC)
Y = Yield (%)
To achieve optimal regression results from the available data, minimize the sum of squared residuals. For the validity (validation) of the model, the coefficient of determination R2 is calculated as follow:
R2 = (St – Sr) / St (1)
Where,
R2: Coefficient of determination
St: the total sum of squares in the dependent variable before regression is performed.
Sr: the sum of squared residuals around the regression line.
Two-factor completely randomized factorial design
The completely randomized design represents the most straightforward form of experimental design. Typically, this design is applied to experiments conducted in a uniform or homogeneous experimental medium or environment [27]. The location of the experiment did not exert a significant influence on the observed responses. The model for the completely randomized design is outlined.
Calculation of pyrolysis performance
- Calculation of the optimal reactor temperature of 300-600 °C.
- Heating rate: the rate of increase in temperature per minute is expected to be 5-200 °C/min.
- Length of combustion time (retention time)
- The calculation of the yield of bio-oil, biochar, and gas can be seen in equations (2),(3), and (4).
e. The data taken in this study were the average solar radiation (I), wind speed, reactor temperature (Tin), and exit temperature at the reactor (Tout). The measuring instruments used in this research were solar power, anemometers, high-temperature thermometers, thermocouples of type K, and digital scales.
f. Energy calculations in solar pyrolysis involve determining the amount of energy the solar collector receives. As explained by Pikra et al. [26], this calculation can be seen in the equation below, which represents the energy produced by the solar collector (Qc).
Results and discussion
Parabolic solar pyrolysis design
The design of this pyrolysis reactor adopts the configuration of a parabolic solar pyrolysis system, representing a relatively recent model in the pyrolysis process. Indeed, researchers commonly employ parabolic troughs and parabolic dishes integrated with fixed-bed batch reactors. The success of a solar pyrolysis process is contingent not only upon the reactor configuration, but also on various process variables [28]. This innovative system employs a parabolic concentrator to generate heat energy for combustion within the pyrolysis reactor. While traditional pyrolysis processes rely on high temperatures produced through the combustion of wood or gas, solar pyrolysis harnesses sunlight by using a parabolic reflector to concentrate solar energy at a focal point. In this research, a dish parabola, shaped like a trough by applying three-dimensional parabolic geometric properties, is employed. Direct beam radiation is reflected and focused onto a focal point receiver, enabling the system to reach operating temperatures exceeding 1000 °C. Typically, concentrators crafted from thin glass materials exhibit a reflectance of 93.5%. The reflectance value should be increased to 95%, and efforts should be made to either transition to polymer materials or implement aluminum coating techniques [29].
The concept behind a parabolic concentrator is to capture incoming light, direct it towards the receiver through reflection, and concentrate it at a single focal point on the concentrator [30]. The refractive index influences the concentrator's optical efficiency and acceptance angle [31]. The primary advantage of employing a parabolic concentrator lies in its ability to achieve higher geometric concentrations within a smaller focal plane [32]. Parabolic dish systems are recognized for offering the highest potential solar conversion efficiency among all CSP technologies, given their direct orientation toward the sun. A pyrolysis reactor decomposes organic compounds through heating without direct contact with external air, maintaining a temperature range of 300 °C to 600 °C. This reactor is instrumental in assessing heat transfer quality, gas and liquid phase processing times, and the yield of the main product [33-34]. Fusing biomass and solar pyrolysis technologies utilize these low-density energy feed stocks to generate high-energy-density fuel [35]. Solar pyrolysis follows the same principles as conventional pyrolysis, utilizing external heat energy, but distinguishes itself by leveraging concentrated solar energy to heat the pyrolysis reactor system [36]. Solar energy utilization proves economically advantageous, reducing production costs for the pyrolysis process through the concentrated solar energy approach. Solar pyrolysis could cut production costs by one-third [37-38]. The solar pyrolysis apparatus is designed to incorporate a solar concentrator as a parabolic dish, achieving a focused energy efficiency of 73-75%. Refer to Figure 3 for the model of the pyrolysis device and its circuit.
Parabolic solar pyrolysis is designed but can produce quite high heat energy. The components of this tool are a parabolic concentrator, pyrolysis reactor, reactor stand, smoke pipe, liquid pipe, condenser stand, and Arduino system. A simple solar pyrolysis system includes a solar reactor, solar collector, condenser, and supporting elements. The specified parabolic collector has an inside surface made of reflecting silver-mirror material, an opening diameter of 2.2 m, a depth of 0.4 m, and a focal distance of 0.75 m. Solar radiation is focused and reflected by this coating onto a receiver at the concentrator's focal point [36,39]. The selection of materials for constructing pyrolysis reactors and CSP is based on the following criteria:
- The material chosen for the pyrolysis reactor is 2 mm-thick stainless steel. Stainless steel was selected due to its properties, including corrosion resistance, high-temperature resistance, and resilience against water and air. Stainless steel, particularly Type 304, is an alloy known for its versatility, widespread use, good chemical composition, mechanical strength, weldability, and corrosion resistance at a reasonable cost. This material forms a thin passive layer that quickly reforms if scratched, eliminating the need for special metal protection. The cylindrical shape of the pyrolysis reactor is deemed most effective for bio-oil production.
- The concentrator design is a parabolic dish, utilizing a 1 mm-thick stainless steel mirror plate. The choice of stainless steel with this thickness considers ease of application and the formation of optimal curvature for a perfect reflection of power to the focal point. The reflector, resembling a concave mirror, depends on the number of curved points. The parabolic reflector follows an asymmetric concentrator system positioned on a horizontal surface, ensuring radiation collection from every angle.
This device can generate combustion temperatures ranging from 300 °C to 650 °C. The attainment of this temperature range has been influenced by several factors, including:
Impact of wind speed
The temperature produced by parabolic solar pyrolysis is significantly affected by wind speed. Higher wind speeds lead to lower temperatures, rendering them inadequate for heat energy production. During the testing process, the wind speed ranged from 0.1 to 0.4 m/s, and this particular wind speed did not substantially impact the temperature generated by the parabolic concentrator. Wind speeds exceeding 0.6 to 4 m/s can reduce temperatures by more than 40%. It is important to note that wind speed inversely affects the resulting temperature, with higher wind speeds leading to lower temperatures and vice versa.
Influence of light intensity
The temperature produced at the focal point of sunlight by a parabolic reflector is significantly influenced by light intensity. A direct correlation exists between higher light intensity and elevated temperature [40-41]. Since sunlight intensity varies daily and cannot be predicted, the temperature fluctuates daily. For instance, a light intensity of 880-1046 W/m2 corresponds to a temperature range of 500-550 °C, while an intensity of 995-1090 W/m2 results in a temperature range of 550–650 °C. Similarly, a light intensity of 819–923 W/m2 produces.
temperatures in the range of 300-400 °C, 857-945 W/m2 yields temperatures of 400-500 °C, and 963-1023 W/m2 leads to temperatures above 500 °C. The impact of light intensity on resulting temperatures is illustrated in Figure 4, with observations conducted from July 27 to August 9, 2022.
Effect of parabolic size
The size and surface area of the parabolic dish are pivotal factors in determining the temperature generated in the pyrolysis reactor. Larger parabolic dishes lead to higher temperatures. The particular parabolic dish examined has a diameter of 2.26 meters and a parabolic height of 0.32 meters, enabling the production of temperatures ranging from 300 °C to 650 °C.
Effect of receiver material
The material chosen for the satellite dish's receiver is a 1 mm stainless steel mirror plate. This selection significantly influences the temperature generated by the satellite dish. Studies suggest mirrors result in lower temperatures than stainless steel mirror plates. Furthermore, opting for a stainless steel mirror as a reflector is preferable due to its ease of installation and superior performance compared to glass.
Effect of reactor materials
The material chosen for constructing the pyrolysis reactor is 2 mm stainless steel. This decision was driven by the material's ability to absorb and transfer exceptionally high heat, thereby expediting the combustion process of raw materials within the reactor. Stainless steel is corrosion-resistant, ensures longevity, and serves as an excellent conductor of heat.The pyrolysis process, operating within the temperature range of 300-650 °C, produces bio-oil as its primary output rather than gas or charcoal. Utilizing solar thermal pyrolysis shows significant potential for addressing global energy needs. However, several challenges should be overcome, including insufficient heat energy distribution within the pyrolysis reactor system, significant heat loss due to air convection over the reactor surface, and concerns regarding the compatibility of reactor materials. An examination of the integration of solar energy with pyrolysis indicates that it can fulfil approximately 47% of the annual energy requirements of the pyrolysis reactor [42-43]. The effectiveness of this process is influenced not only by the choice of feedstock and reaction dynamics but also by the solar-thermal system and reactor configuration [44]. According to Joardder et al. [45], the advantages of employing solar pyrolysis are as follows:
- High heat flux facilitates rapid heating of the pyrolysis reactor to elevated temperatures.
- It diminished secondary reactions in various regions of the heating chamber due to the relatively small focus area.
- Renewable heat sources reduce the heating costs of pyrolysis reactors and safeguard non-renewable energy reserves.
- The absence of fossil fuel combustion results in a system that produces no emissions, rendering it environmentally sustainable.
- There is no contamination of pyrolysis gas by combustion products, resulting in improved yield quality.
- Implementation of a relatively straightforward heating system
Solar pyrolysis operational parameters
Various parameters significantly impact the performance of solar pyrolysis, encompassing the treatment of raw materials. This treatment involves drying and reducing the size of coconut shells, considering factors such as moisture content, light intensity, temperature, reactor type, heating rate, residence time, and pyrolysis temperature. In the pyrolysis technique, several parameters exert influence on the pyrolysis process, including initial biomass treatment, water content, particle size, biomass compound composition, temperature, heating rate, gas flow rate, residence time, type of pyrolysis, type of pyrolysis reactor, and final pyrolysis product [46-47]. The operational parameters used to calculate solar pyrolysis performance include:
Pre-treatment of raw materials
The initial treatment of coconut shells involves a drying and size reduction process, impacting the quality of the bio-oil produced and the performance of diesel pyrolysis. According to Hassan et al. [48], the initial treatment of biomass in the fast pyrolysis process was demonstrated to alter the structure and chemical composition, leading to changes in the thermal decomposition mechanism. The enhancement of biomass raw material quality and its pyrolysis products frequently necessitates biomass pretreatment [49].
The coconut shell undergoes cleaning and crushing to achieve smaller sizes using a shell grinder. Subsequently, it is dried for two weeks at temperatures ranging from 60 °C to 80 °C to achieve a moisture content of 8-11%. Lower material moisture content expedites the combustion process in the reactor, resulting in superior bio-oil and biochar quality.
Material with a moisture content exceeding 12% leads to higher water content in the bio-oil, thereby prolonging the purification process. Biomass drying, facilitated by a hot air flow at 150°C, effectively reduces water content [50-51]. Reduced water content accelerates thermochemical decomposition, enhancing
bio-oil production and essential compound yields such as phenol during pyrolysis [52].
Elevated temperatures significantly decrease material water content [53]. Following the drying process, the coconut shell is reduced using a grinder machine, resulting in material sizes of 10 mm, 5 mm, and 3 mm, which are subsequently introduced into the pyrolysis reactor. Field research data reveals that 10 mm and 5 mm coconut shell sizes exhibit higher flammability than finer raw materials. Material size notably influences the combustion process in the pyrolysis reactor, as evidenced by shorter pyrolysis times for larger material sizes.
Typically, material particle sizes used after cutting are 10-30 mm, and after grinding, they range from 0.3 to 1.5 mm [54]. Shen et al. [55] assert that a smaller material particle size (0.3-1.5 mm) can increase bio-oil production. However, contrary to several articles suggesting that raw material sizes of 10 mm and 5 mm are more flammable than fine or very fine, this study found that fine material sizes require a longer combustion time. If the burning time is equivalent to larger material sizes, most fine material sizes may burn partially, necessitating a longer residence time. The coconut shell sizes are depicted in Figure 5.
Temperature
Temperature is crucial in influencing the pyrolysis process to achieve optimal results. Its function is to provide the necessary heat for breaking down the chemical bonds in biomass. Temperature emerges as the most critical factor influencing product yields. However, existing literature needs to be more dispersed, precise, and challenging regarding the system configuration and operational parameters that impact solar product yield [28]. The temperature range produced by the parabolic solar pyrolysis concentrator is 300-650 °C. The resultant high or low temperatures depend on the intensity of sunlight and wind speed. Higher sunlight intensity leads to higher temperatures, while stronger wind results in lower temperatures, even in high sunlight. Temperature is a crucial factor in determining how efficiently biomass compounds decompose because an increase in temperature speeds up the process of breaking bonds. Numerous studies indicate that temperatures between 450 °C and 550 °C yield high bio-oil, though this value can vary based on the type of biomass used and other process variables [56].
Heating rate
The heating rate plays a pivotal role in determining the final product of the pyrolysis process, influencing the properties and composition of the end product. A low heating rate reduces the pyrolysis reaction, preventing biomass's thermal breakdown, resulting in more charcoal than bio-oil. The heating rate in this process ranges from 20 to 150 °C/min.
Higher heating rates accelerate the decomposition process of biomass compounds, leading to increased production of bio-oil and gas [57]. Secondary pyrolysis at high heating rates aids in the formation of gas components. Conversely, a low heating rate is associated with a higher charcoal yield [58]. Uzun et al. [59] researched soybean meal pyrolysis using a fixed bed reactor, finding that a higher heating rate resulted in a significant increase in bio-oil yield, with a 23.36% increase observed by raising the heating rate from 5 to 700 °C/minute.
Residence time
The residence time of biomass material in a reactor is a crucial factor influencing the decomposition of oil and gas. A longer residence time generally leads to higher bio-oil yields. Mohamed et al. [60] and Tsai et al. [61] highlight that residence time significantly influences the composition of bio-oil and gas compounds, though its impact on charcoal yield is less pronounced. Incomplete re-polymerization of biomass components and small charcoal production result from very short residence times for steam [62].
The influence of residence time on the decomposition of solid biomass material into oil and gas is significant. Lower temperatures result in longer residence times, leading to higher charcoal production. In the fast pyrolysis of poplar wood, an increase in charcoal production is observed with longer residence times [63-64]. However, it is essential to note that temperature, heating rate, and other parameters often dominate residence time.
Mathematical model of the effect of temperature and particle size on results
The data influencing the results include temperature and particle size, each with three repetitions. Temperature will be measured at 300 °C, 400 °C, 500 °C, and 600 °C, while particle sizes are 3 mm, 5 mm, and 10 mm. Combination of these two factors will serve as the basis for the mathematical model equation in this research. This mathematical model provides an overview of the resulting yield (Y) due to the interaction between material size factors (X1) and temperature (X2). The interaction between these two factors allows calculating coefficient values a0, a1, and a2, which influence the mathematical model. The parameters influencing the speed of the pyrolysis reaction have a very complex relationship. Consequently, each researcher's mathematical model for the pyrolysis reaction speed equation always exhibits a different empirical formulation [65]. The form of the mathematical equation with multiple linear regression involving two independent variables is:
The goal is to minimize the sum of squared residuals to obtain the best regression results from the available data. Regression analysis demonstrates the performance of the regression model on the utilized data conducted using the SPSS program.
The value of the regression analysis R2 is 0.64 (64%), with a Standard Error of 2.6778.
The R2 value ranges from 0 to 1, with higher values indicating a better model for explaining the data used. R2 value of 64% indicates that the particle size of the material and the temperature used influence the resulting yield, while other factors influence 36%. Standard Error estimates the standard deviation of the model's prediction error, and a smaller value indicates a better model at predicting the data.
Calculation of a complete random design two factorials
This research used two treatments (particle size and temperature), constituting all possible combinations of factor levels for the tested factors. Factor "a" represents the size of the coconut shell material, and factor "b" represents the temperature. The completely randomized design is the simplest type of experimental design employed for experiments characterized by uniform or homogeneous conditions. The research obtained experimental data with 12 combinations of factors "a" and "b," as provided in Table 1.
After calculating the average of the combined treatment data, the next step is to analyze the data using a completely randomized design and analyze the variance. The analysis of variance involves calculating the correction factor (CF), the total sum of squares (TSS), the sum of treatment squares (STS), the sum of error squares (SES), the treatment mean square (TMS), the error mean square (EMS), determining the F-value, calculating the F table for 5% and 1%, and drawing conclusions based on the analysis results. The calculations for these values are presented in Table 2.
Table 2 indicates that within treatment columns A and B, the F-count value exceeds the F-table values at the 5% and 1% significance levels. Thus, it is evident that the treatment factors of material size (A) and temperature (B) significantly influence the results (yield). In addition, the combined treatment of A and B, indicated with one star, significantly impacts the results (yield). This is evident from the F-count value being more significant than the F-table value at a 5% significance level but smaller than that at a 1% significance level.
The Duncan test
The Duncan test is an additional analysis used to distinguish between similar and dissimilar middle values when assessing the homogeneity of multiple data points. This test is precious when the null hypothesis is rejected and the alternative hypothesis is accepted. Furthermore, the Duncan test sheds light on the mean value of the treatment when significant differences exist. Applying the Duncan test with a significance level of 0.05 makes it possible to pinpoint the treatment shares that demonstrate noteworthy variations. Duncan's test to determine the effect on particle size is listed in Table 3.
Table 3 shows no significant difference in average yields between the 3 mm and 5 mm material sizes. In contrast, the 10 mm material size yields different results than the other two. The impact of temperature differences
Referring to Table 4, it is evident that the temperature treatments of 500 °C and 600 °C resulted in similar average yields. In contrast, the treatments at 300 °C and 400 °C exhibited a significantly different average yield compared to the temperatures at 500 °C and 600 °C.
The effect of temperature and particle size on pyrolysis yield
Pyrolysis performance calculations were conducted to assess the quantity and quality of results obtained using solar pyrolysis equipment on bio-oil yield. The performance on yield can be observed in Table 4.
Calculation considers data such as temperature and particle size, with the anticipated outcome being an increased bio-oil yield. This research evaluates the performance of the pyrolysis process by comparing the yields of bio-oil, biochar, and gas under varying temperatures and particle sizes.
Figure 6 provides a visual representation of the impact of temperature on yield. At a temperature of 300 °C, a particle size of 3 mm yields 26.83%, while a particle size of 5 mm yields 29.33%, and a particle size of 10 mm yields 25.33%. When the temperature is increased to 400 °C, a particle size of 3 mm yields 30.43%, 5 mm yields 27.5%, and 10 mm yields 26.33%. At 500°C, 3 mm particle size yields 35.17%, 5 mm yields 34.5%, and 10 mm yields 26.33%. Finally, at 600 °C, a 3 mm particle size yields 37.67%, 5 mm yields 32.17%, and 10 mm yields 30.33%. Analyzing this data leads to the conclusion that a particle size of 3 mm and a temperature of 600 °C yield the highest results. Generally, smaller material sizes and higher temperatures contribute to maximize results. To determine the effect of temperature on yield (Figure 7).
Based on Figure 7, the particle size of 3 mm yields the highest results, specifically at temperatures of 500 °C and 600 °C, namely 35.17% and 37.67%, respectively. Meanwhile, for the particle size of 5 mm, the highest yield value was obtained at a temperature of
500 °C. For the particle size of 10 mm, the highest yield occurred at 600 °C, reaching 30.33%. Analysis of this data indicates that the smaller the size of the material, the higher the yield produced. Temperatures between 500 °C and 600 °C provide the best results in testing parabolic solar pyrolysis. The bio-oil yield reached 20%, with biochar and pyrolytic gas at 51% and 29% respectively, but averaged 12% due to heat transfer losses, catalyst absence, and solar flux distribution [66-67]. Rice husk was subjected to solar pyrolysis, which mainly produced bio-oils, ranging in weight from 20.6% to 43.13%, with biochar and pyrolysis gases as byproducts. The amount of pyrolysis gas produced increased linearly with temperature, from 14% at 500 °C to its maximum yield of 25.48% at 800 °C [68].
Calculation of energy in parabolic solar pyrolysis
The calculation of the amount of energy required to use concentrated solar energy can be adjusted according to the technology and the conditions and systems being used. There are several steps to this calculation, including:
- Computation of the parabola's area: With a base length of 2.26 m and a height of 0.32 m, the area of the parabola, as per formula (4), amounts to 0.482 m².
- The intensity of concentrated sunlight is the amount of solar energy received per unit area capturing sunlight in a particular time, in units of W/m2. The intensity of sunlight varies depending on geographic location, weather conditions, and the orientation of the light catcher.
- The calculation of the energy received by the system (input) involves determining the heat energy generated by the solar concentrator and the energy within the pyrolysis reactor, employing formulas (5), (6), and (10). The input energy calculation can be seen in Figure 8 (a) Q collector and (b) Q reactor.
The direct heating reactor is the predominant heating method in solar pyrolysis systems. Typically constructed from a quartz tube, this reactor features a transparent window exposed to concentrated solar radiation, directing it onto the feed stocks for decomposition [69-70]. The input heat energy is derived from the summation of the heat energy produced by the concentrator and the heat energy present in the pyrolysis reactor. The heat energy produced by the concentrator is calculated as the product of the parabola's area and the intensity of sunlight. Notably, the concentrator's energy is less than the heat energy within the reactor. This discrepancy arises from the concentration of heat energy at a single focal point in the pyrolysis reactor. Specifically, the heat energy produced by the parabolic collector ranges from 394.76 to 525.38 W, whereas the heat energy received by the pyrolysis reactor ranges from 2000.80 to 6677.98 W.
The calculation of the energy released by the system (output) entails assessing the heat energy generated by the solar concentrator and the energy within the pyrolysis reactor using formulas (7), (8), (9), and (11). The output energy calculation can be seen in Figure 8 (c) Q biomass and (d) Q loss. Figure 8 shows that the energy that influences solar pyrolysis has a linear regression equation (0.80-0.99).
The temperature acquired at the focal point at the bottom of the reactor is measured using a K-type thermocouple (T0). The temperature within the reactor is measured with a digital thermometer (T1). In contrast, the temperature at the top of the reactor is noted as (T2), and the temperature at the top of the reactor lid is recorded as (T3).
The heat energy received by the coconut shell ranges from 136.29 to 454.89 W and the energy lost during pyrolysis, specifically between 728.46 and 1778.976 W. The output heat energy is obtained from the sum of the heat energy received by the coconut shell and the heat energy lost in the reactor. The output heat energy ranges from 864.75 to 2233.87 W, depending on the temperature difference in the reactor. The primary heat loss is caused by biomass reflectivity (37.85%) and the difference in environmental and reactor temperatures (36.23%) [17]. The output heat energy ranges from 864.75 to 2233.87 W; this depends on the temperature difference in the reactor.Energy conversion efficiency is the ratio between the output energy produced by the system and the input energy (received solar energy). Energy conversion efficiency can vary depending on the type of used technology. Calculating heat energy efficiency in concentrators and pyrolysis reactors during performance testing can be determined by dividing the heat energy output by the input.
Energy efficiency calculations are performed by comparing the output energy to the input energy, multiplied by 100%. The energy efficiency obtained during this research varies depending on the factors and parameters influencing the solar pyrolysis process. Energy efficiency ranges from 16.35% to 30.41%. The heat balance is analyzed by applying the principles of optics and thermodynamics.
Conclusion
Energy is a crucial political issue due to fuel and economic crises in many countries. Renewable energy sources include carbon-based biomass from biological sources like agricultural leftovers and forest remnants. Coconut shells have significant potential for transforming into a renewable energy source through pyrolysis, generating value-added products. The pyrolysis process, which converts biomass waste into solid fuel and syngas, and also bio-oil is a promising alternative. This study uses concentrated solar energy to generate heat through a parabolic collector, a dish-like shape formed by applying a three-dimensional parabola to a trough-like design. The pyrolysis reactor design uses a parabolic solar pyrolysis system, a recent model. The solar pyrolysis apparatus incorporates a solar concentrator as a parabolic dish, achieving 73-75% focused energy efficiency.The parabolic solar pyrolysis device can generate combustion temperatures ranging from 300 to 650 °C, influenced by wind speed, light intensity, parabolic size, receiver material, and reactor materials. Various parameters, including the treatment of raw materials, such as coconut shells, significantly influence the performance of this process. These parameters include moisture content, light intensity, temperature, reactor type, heating rate, residence time, and pyrolysis temperature.The research used two treatments (particle size and temperature) to analyze the effects of coconut shell material size and temperature on results. The completely randomized design resulted in 5% and 1% significance levels. The results showed that the treatment factors of material size and temperature significantly influenced the results, and the combined treatment of A and B also significantly impacted the results. To evaluate the performance of solar pyrolysis equipment on bio-oil yield by comparing yields of bio-oil, biochar, and gas under varying temperatures and particle sizes. Results show that a particle size of 3 mm and a temperature of 600 °C yield the highest results. With smaller material size, the higher yield is produced. The study concludes that temperatures between 500 °C and 600 °C provide the best results in testing parabolic solar pyrolysis. The results suggest that smaller material sizes and higher temperatures maximize pyrolysis results. The calculation of the energy required for concentrated solar energy depends on the technology and conditions used. The process involves calculating the area of a parabola, the intensity of concentrated sunlight, and the heat energy generated by the solar concentrator and the pyrolysis reactor. The direct heating reactor is the predominant heating method in solar pyrolysis systems. The input heat energy is derived from the sum of the heat energy produced by the concentrator and the heat energy present in the reactor. The output heat energy is calculated from the sum of the heat energy received by the coconut shell and the heat energy lost in the reactor. The energy conversion efficiency is the ratio between the output energy produced by the system and the input energy. The energy efficiency varies depending on the factors and parameters influencing the solar pyrolysis process, ranging from 16.35% to 30.41%.
Acknowledgments
I would like to thank the research supervisor team and all parties who have assisted in this research.
Conflict of interest
Authors declares there is no conflict of interest in this research
Data Availability
The data presented in this research are available on reasonable request from the corresponding author.
Orcid:
Sri Aulia Novita: https://orcid.org/0000-0002-2585-0082
Santosa: https://orcid.org/0000-0002-2019-8697
Nofialdi: https://orcid.org/0000-0001-8509-4242
Andasuryani: https://orcid.org/0000-0003-3661-8075
Ahmad Fudholi: https://orcid.org/0000-0002-9528-7344
Azril: https://orcid.org/0000-0001-8685-5517
Rahadian Zainul: https://orcid.org/0000-0002-3740-3597
----------------------------------------------------------------------------------------
How to cite this article: Sri Aulia Novita, Santosa , Nofialdi , Andasuryani, Ahmad Fudholi, Azril, Rahadian Zainul .Parabolic dish solar pyrolysis for bio-oil production: performance and energy analysis. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(1), 89-110. Link: http://jmpcr.samipubco.com/article_183498.html
----------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
