Document Type : Original Research Article
Authors
1 Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia
2 Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Airlangga - Dr. Soetomo General Academic Hospital, Surabaya, Jawa Timur, Indonesia
Abstract
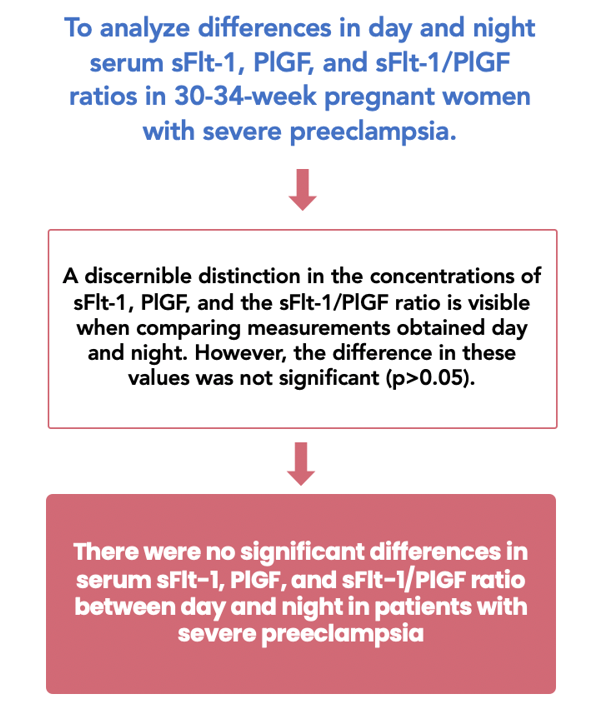
Preeclampsia is identifiable by an imbalance of angiogenic factors, which entails elevated levels of sFlt-1 and a reduction of PlGF levels. In addition to disruptions in circadian rhythms, preeclamptic individuals may exhibit daytime and nighttime alterations in sFlt-1, PlGF, and the sFlt-1/PlGF ratio. This study aims to assess the differences in daytime and nighttime concentrations of sFlt-1, PlGF, and the sFlt-1/PlGF ratio in severe preeclamptic women between 30 and 34 weeks of gestational age. This cross-sectional study was conducted at Dr. Soetomo Public Academic Hospital and Airlangga University Hospital in Surabaya, involving ten pregnant women at 30-34 weeks of gestation, all diagnosed with severe preeclampsia. Samples of blood were drawn in the morning at 08:00, followed by the evening at 20:00. The study found no significant differences in serum levels of sFlt-1 (5.99 ± 3.29 vs. 6.82 ± 4.09 pg/mL), PlGF (85.5 [37-312] vs. 72 [35-437] pg/mL), and the sFlt-1/PlGF ratio (48.5 [6-233] vs. 58.5 [3-345] pg/mL) between day and night (p > 0.05). The results suggest that the circadian rhythm does not notably influence the serum levels of anti- and proangiogenic substances in confirmed severe preeclampsia.
Graphical Abstract
Keywords
Introduction
Preeclampsia is hypertension that develops at or after the twentieth week of pregnancy, along with urine protein, organ failure, and uteroplacental impairment. This condition ranks as one of the most significant global problems involving the deaths of pregnant women. It causes approximately 76,000 fatal maternal deaths and 500,000 deaths of newborns annually [1-4]. Preeclampsia is a "disease of theories". The aberrant placentation hypothesis is the most accepted currently. The stress of syncytiotrophoblast and endothelial tissue cell reactivity are the core features of the pathognomonic process, significantly impacting the angiogenic substances involving Placental Growth Factor (PlGF) and Soluble Fms-like Tyrosine Kinase 1 (sFlt-1), leading to an angiogenic imbalance and subsequent manifestation of the clinical hallmarks of preeclampsia [5-9].
PlGF represents a proangiogenic factor, while sFlt-1 represents an antiangiogenic factor. In preeclamptic patients, PlGF levels decrease, and sFlt-1 levels increase, resulting in an elevated sFlt-1/PlGF ratio [10-14]. Several countries extensively utilize this biomarker for the prediction, diagnosis, and management of preeclampsia [12,13,15,16].
Antiangiogenic and proangiogenic levels and ratios might fluctuate day and night. Cortisol and melatonin could impact alterations in biomarkers. Circadian rhythms physiologically influence these hormones [17-21]. Preeclamptic patients could disrupt those hormone releases due to circadian rhythm abnormalities [22-24].
This study suggests alterations throughout the day and night in anti- and proangiogenic factors and their ratios in severe preeclamptic patients. Previous studies randomly obtained blood samples for these biomarkers at various times of the day, even over weeks, without emphasizing a specific sampling time [25,26]. No study has investigated the daytime and nighttime alterations and characteristics of sFlt-1, PlGF, and the ratio of them in severe preeclamptic pregnancy nationwide. Thus, this pilot study is essential to determine day-to-night fluctuations in those angiogenic factors in confirmed severe preeclampsia at 30-34 weeks gestation.
Methods
This cross-sectional study was conducted at the tertiary hospitals Dr. Soetomo Public Academic Hospital and Airlangga University Hospital in Surabaya. It involved ten pregnant women at 30 to 34 weeks of gestation who met the specified inclusion and exclusion criteria. The inclusion criteria encompassed singleton pregnant women diagnosed with severe preeclampsia between thirty to thirty-four weeks of gestation, receiving expectant management. In contrast, the exclusion criteria included labor conditions, placenta accreta spectrum, malignancy during pregnancy, gestational and pregestational diabetes mellitus, and superimposed chronic hypertension. These women were diagnosed with severe preeclampsia based on the criteria set forth by the International Society for the Study of Hypertension in Pregnancy (ISSHP) [3].
Participants were selected using a sequential sampling method. To determine the level in the serum of sFlt-1, PlGF, and their ratio, venous blood specimens were subsequently drawn at 8:00 and 20:00. The chosen timing, aligned with the circadian rhythm, was intended to capture potential fluctuations in cortisol and melatonin hormone levels. While this study did not measure confounding factors such as anxiety during pregnancy, it is acknowledged that these factors might impact hormone levels [18,27]. The analysis of angiogenic factors utilized Roche Elecsys®. The Elecsys reagent kit and COBAS e601 analyzer's Electrochemiluminescence Immunoassay (ECLIA) were utilized to calculate the serum sFlt-1 and PlGF levels. The Elecsys PlGF ranges from 3 to 10,000 pg/mL, with levels below three categorized as < 3 and excess values as > 10,000. Elecsys sFlt-1 ranges from 10 to 85,000 pg/mL, with levels less than ten classified as < 10 and values higher than eighty-five thousand as > 85,000 [28,29].
The assessment of day-and-night serum anti- and proangiogenic factors commenced with a normality test using the Shapiro-Wilk test. The hypotheses were validated with a paired t-test. Version 25 of SPSS was used for the analysis of statistical data. For normally distributed data, the paired t-test was utilized, while the Wilcoxon signed-rank test was employed for data with abnormal distributions. The significance level for this pilot study was set at 0.05, indicating significance if p < 0.05 from the statistical test.
Results
A total of ten patients participated in this pilot study, with six at Dr. Soetomo Public Academic Hospital and four at Airlangga University Hospital. The average body mass index of the patients recruited for this study was 33.71 kg/m², while their mean age was 32.9 years (Table 1).
According to this study, the average expectant woman diagnosed with severe preeclampsia is obese. In addition, 50% of patients in this study were primiparous, and the other 50% were multiparous. The median gestational age of patients presenting for the first time was 31 weeks. An analysis of variance was conducted on the daytime and nighttime serum levels of sFlt-1, PlGF, and their ratio among severe preeclamptic pregnancies between thirty and thirty-four weeks of gestation.
The average serum sFlt-1 concentration was 5,992 ± 3,291 pg/mL during the day and 6,817 ± 4,088 pg/mL at night. Table 2 revealed that both comparable periods were not significantly different (p = 0.063).
The median morning serum PlGF level was 85.5 pg/mL, while the nighttime serum PlGF level was 72.0 pg/mL. Table 3 illustrates an insignificant difference in serum PlGF values throughout the two phases of the day (p = 0.445).
The median value for the morning sFlt-1/PlGF ratio group was 48.5, while it was 58.5 for the night group. There was no significant difference between the daytime and nighttime ratios of sFlt-1/PlGF (p = 0.241), as depicted in Table 4.
Discussion
The preliminary investigation attempted to evaluate the fluctuation in sFlt-1 and PlGF levels throughout the day and night in severe preeclampsia. However, the serum concentrations of proangiogenic and antiangiogenic substances did not differ significantly between the periods of the day and night. This outcome might occur due to the confounding factors influencing the concentration of these biomarkers.
Theoretically, in a normal pregnancy, blood pressure displays a diurnal rhythm, exhibiting higher daytime than nighttime levels, akin to non-pregnant patterns. Nighttime typically sees a 10-20% reduction in blood pressure, termed "normal dipping." Conversely, women with preeclampsia often experience "reverse dipping," where blood pressure exceeds daytime levels due to disrupted circadian rhythms. Reports have highlighted disruptions in placental circadian rhythms in preeclamptic women [9,23,30-32]. Maternal hormonal abnormalities may disrupt circadian rhythms and contribute to pregnancy complications. Cortisol and melatonin are the primary influences on circadian rhythms and are vital to this mechanism [22,24,33-35].
The adrenal gland releases cortisol in a circadian rhythm, resulting in elevated morning cortisol levels and a gradual decline in nocturnal levels. Disruptions in cortisol secretion lead to fluctuations in daytime and nighttime exposure [24,36-38]. The pineal gland is responsible for the production of lipophilic melatonin (5-Methoxy-N-Acetyltryptamine). However, the placenta produces a great deal of it during pregnancy. The placenta produces more melatonin than the pineal gland- the normal melatonin levels during pregnancy peak at night and decline during the day. Abnormalities in melatonin secretion influence the maternal circulation of melatonin [23,33,39-41]. These circumstances might influence the placental synthesis of angiogenic factors, potentially altering their serum levels and ratios throughout the day and night. This phenomenon, driven by disrupted circadian rhythms in severe preeclampsia, results in a reverse dipping effect, causes an increase in blood pressure during the night compared to daytime levels [22-23,31,42]. Hypertension, a manifestation of preeclampsia due to endothelial dysfunction resulting from an imbalance in angiogenic factors, tends to worsen at night. This exacerbation may lead to elevated sFlt-1 and PlGF levels at night, consequently impacting the increased sFlt-1/PlGF ratio. Elevated nocturnal blood pressure could potentially lead to kidney and cardiac damage [9,17,43-45]. This study reveals a gap in the analysis of antiangiogenic and proangiogenic factors and their ratio between day and night, but no differences in sFlt-1, PlGF, and the ratio of them in severe preeclamptic patients using statistical analysis. Supposedly, this may occur due to confounding factors. Previous studies collected blood samples for this biomarker randomly throughout the day and even weekly, lacking specific guidelines for the precise timing of biomarker measurements [25,26].
This study demonstrated that biomarker testing might be performed at any time during the day. However, it emphasizes the necessity of more extensive research to determine the most suitable timing for obtaining biomarker blood samples to ensure accuracy. Furthermore, the relationship between circadian rhythms and anti- and proangiogenic factors demands further research. Several limitations are evident in this study. First, the study included only ten participants from two specific tertiary hospitals, limiting the generalizability of the findings to a broader population. The small sample size may not adequately represent the diverse characteristics of severe preeclampsia patients. Second, the study assumed a consistent circadian rhythm impact on biomarker levels without accounting for potential variations in individual circadian patterns among participants.
Conclusion
Serum concentrations of sFlt-1, PlGF, and their ratio did not fluctuate drastically day and night among patients diagnosed with severe preeclampsia. The angiogenic factors could be examined at any time of the day. The precise timing for collecting a blood sample for this biomarker should be investigated in greater depth. In addition, the effect of cortisol and melatonin substances on diurnal and nocturnal fluctuations in antiangiogenic and proangiogenic biomarkers requires further investigation.
Acknowledgements
The authors intend to acknowledge the entire hospital staff for participating in patient care and completing this study.
Funding
There were no specific grants from public, private, or non-profit funding agencies for this research.
Authors’ contributions
All authors (D.W.P. and M.I.A.A.) contributed in some manner to this research, involving conceptualization, design, interpretation, drafting of the manuscript, and critical revision of substantial intellectual content. All authors have reviewed and approved the manuscript in its final form.
Conflict of interest
The authors declare no conflict of interest
Orcid:
Dewangga Wahyu Praja: https://www.orcid.org/0009-0009-7319-5578
Muhammad Ilham Aldika Akbar: https://www.orcid.org/0000-0002-2003-9282
---------------------------------------------------------------------------------------------
How to cite this article: Dewangga Wahyu Praja, Muhammad Ilham Aldika Akbar*, The differences of serum levels of sFlt-1, PlGF, and sFlt-1/PlGF ratio throughout daytime and nighttime in women with severe preeclampsia at 30-34 weeks of pregnancy: a pilot study. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(2), 166-173. Link: http://jmpcr.samipubco.com/article_183726.html
---------------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
