Document Type : Review Article
Authors
Department of Ophthalmology, Dr. Soetomo General Academic Hospital, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
Abstract
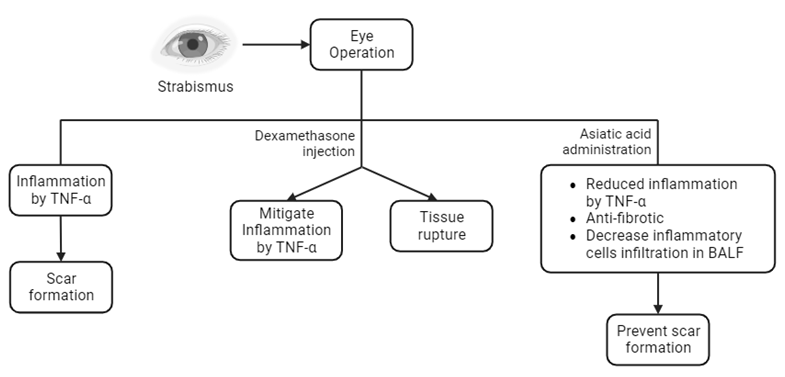
Strabismus, also known as ocular misalignment or crossed eyes, refers to an abnormality characterized by a deviation in the alignment of the eyeballs. Following strabismus surgery, there is a possibility of postoperative inflammation leading to the development of fibrotic tissue. Inflammation elicits a cellular response characterized by the presence of neutrophil cells, subsequently accompanied by the emergence of pro-inflammatory cytokines, such as TNF-α. The occurrence of conjunctival scar is a prevalent local consequence observed in around 90% of strabismus procedures that involve the manipulation of more than one muscle. Dexamethasone is a synthetic adrenal corticosteroid with potent anti-inflammatory effects, mostly inhibiting undesired immune system reactions. The subconjunctival administration of dexamethasone is commonly employed during strabismus surgery to mitigate inflammation. Subconjunctival steroid injection near the affected cornea or sclera can weaken the tissue, which may result in rupture at the injection site. Centella asiatica, commonly referred to as gotu kola leaves in Indonesia, is recognized as a potent botanical agent employed in traditional medicine. Asiatic acid, the primary component of Centella asiatica saponins, exhibits a diverse range of biological actions, including anti-inflammatory, anti-fibrotic, antioxidant, and neuroprotective effects. The administration of Asiatic acid has been found to exhibit an anti-fibrotic action through the inhibition of TGF-𝛽 signaling. In addition, it can decrease the infiltration of inflammatory cells in bronchoalveolar lavage fluid (BALF) and the production of pro-inflammatory cytokines.
Graphical Abstract
Keywords
Introduction
Strabismus, also known as ocular misalignment or crossed eyes, refers to an abnormal condition characterized by the misalignment of the eyeballs. The primary objective of strabismus surgery is to enhance the alignment of the eyeball, hence improving visual function, binocular vision, and cosmetic appearance. Following strabismus surgery, there is a possibility of inflammation leading to the development of fibrotic tissue. One consequence of inflammation is the recruitment of neutrophil cells, which is subsequently accompanied by the release of pro-inflammatory cytokines, such as TNF-α. The presence of an inflammatory process that fails to resolve effectively during wound healing might lead to the development of fibrosis, which can subsequently impede the desired outcome following strabismus surgery [1,2].
Scar remodeling following strabismus surgery is responsible for 50% of cases of overcorrection and 10% of cases requiring reoperation. The occurrence of conjunctival scar is a prevalent local consequence observed in around 90% of strabismus procedures that involve the manipulation of more than one muscle. Consequently, the occurrence of tissue fibrosis gives rise to the development of muscle limits after strabismus surgery. Prolonged strabismus frequently induces alterations in the morphology of the extraocular muscles, leading to muscular degeneration. This degenerative process might provide challenges for surgical intervention, often necessitating the need for multiple surgical procedures. Strabismus recurrence following surgery is a frequent phenomenon that researchers have extensively studied. These investigations have identified several prognostic markers connected with this recurrence, which may necessitate further surgical intervention or prolonged therapeutic measures. The phenomenon above can give rise to social challenges and impede the patient's routine activities [2-5]. Dexamethasone is a synthetic adrenal corticosteroid with potent anti-inflammatory effects, mostly inhibiting undesired immune system reactions. The subconjunctival administration of dexamethasone is commonly employed during strabismus surgery to mitigate inflammation.
Nevertheless, it is widely acknowledged that the administration of dexamethasone is associated with several unanticipated adverse reactions. Ocular hypertension or glaucoma generated by steroid use might manifest in susceptible individuals, particularly following the application of dexamethasone by topical, periocular, or intraocular routes. Dexamethasone injection can induce temporary hyperglycemia in diabetic patients following vitreoretinal surgery. According to literature, it has been shown that administering subconjunctival steroid injections on the affected cornea or sclera may result in tissue thinning and perhaps lead to rupture at the injection site [6,7].
Traditional remedies derived from plants are prevalent among over 80% of the global population, particularly in developing nations, as reported by the World Health Organization (WHO). Centella asiatica, commonly referred to as gotu kola leaves in Indonesia, is recognized as a potent botanical remedy within traditional medicine. Asiatic acid, the primary component of Centella asiatica saponins, exhibits a diverse range of biological actions, including anti-inflammatory, anti-fibrotic, antioxidant, and neuroprotective effects. Nevertheless, the present body of research on the effects of Asiatic acid on ocular health remains restricted [8,9].
Applying topical diclofenac following strabismus surgery might effectively mitigate inflammation at the tendon-sclera attachment site, potentially reducing fibrosis [10]. Triamcinolone acetonide and obtained comparable findings. In recent years, considerable scholarly attention has been devoted to investigating the potential of Asiatic acid as an antifibrotic agent. The effects of Asiatic acid administered orally to mice models of liver fibrosis generated by CC14 through in vivo and in vitro experiments. The results indicated that 8 mg/kgBW of Asiatic acid had an anti-fibrosis impact by inhibiting TGF-𝛽 signaling [11]. Asiatic acid, which is a constituent of Centella asiatica sp., has a significant role in the management of keloids [10]. It can inhibit the infiltration of inflammatory cells in bronchoalveolar lavage fluid (BALF) and reduce the levels of pro-inflammatory cytokines in lung tissue specimens produced by bleomycin. The TGF-𝛽1 expression in lung tissue is suppressed by Asiatic acid, which is also associated with a reduction in type I collagen, type III collagen, and matrix metalloproteinase (MMP) levels [12]. According to Adtani et al. (2016), it was elucidated that the compound known as Asiatic acid exhibits a considerable downregulating effect on arecoline-induced fibrosis in human buccal fibroblasts by inhibiting the signaling pathway of transforming growth factor-beta (TGF-𝛽) [13].
Based on aforementioned notes, researchers aim to investigate the impact of Asiatic acid on the TNF-α expression and the number of neutrophil cells as an inflammatory response in the extraocular muscles after strabismus surgery in rabbit experiments. It will be achieved by implementing Hematoxylin Eosin and Immunohistochemistry (IHC) analyses. Further research is warranted in this area. The rationale behind conducting this research on rabbits stems from ethical and technical constraints that precluded the use of human subjects. The anticipated outcome of this research is that it will serve as the foundation for identifying and implementing alternative therapeutic approaches for patients following strabismus surgery.
Surgery for strabismus
The primary objective of strabismus surgery is to correct the misalignment of the ocular position. This procedure involves many processes, including muscular relaxation and tightening and altering the vector of muscle force by relocating the insertion site of the extraocular muscles. The contraction of the extraocular muscles results in a force that induces the movement of the ocular structures. The torque is linearly related to both the magnitude of the moment arm and the force exerted by the muscles during contraction [14-17].
Muscular recession refers to muscular relaxation, whereby the muscle insertion is brought closer to the muscle's origin [1]. This phenomenon aligns with Starling's law, which posits that a lax muscle will decrease. As the muscle undergoes greater relaxation, there is a corresponding decrease in the force created by the muscle. Furthermore, muscular relaxation is enhanced when the eye shifts its gaze toward the recessed muscle, resulting in a reduction in rotational force as the eye moves in the direction of the muscle. When the gaze turns away from the concave muscle, there is a decrease in muscle relaxation, leading to an increase in rotational force. In individuals diagnosed with right eye esotropia, it has been observed that the degree of misalignment tends to worsen when attempting to gaze toward the left. In such cases, surgical intervention, including the medial rectus muscle recession, effectively reduces the misalignment. When the right eye is directed towards the left, there is an increase in muscular relaxation, resulting in a reduction of rotational force [14-18].
The technique employed to induce muscular shortening involves the surgical procedures of muscle excision, folding, and tucking of the extraocular muscles. These techniques aim to enhance the strength and tension of the extraocular muscles. The contraction of muscles might result in a decrease in the rotation of the eye away from the muscles that have undergone contraction. The occurrence of a shortened right medial rectus muscle can result in an esodeviation that exhibits an increment when the eye is moved towards the right. This condition is typically observed in cases of exotropia that demonstrate an increase in deviation when the eye is moved toward the right [14-18].
Muscle resection is a surgical procedure that involves the excision of the anterior portion of a muscle, followed by suturing the severed ends back to their original insertion site. This method is frequently employed in clinical practice. One approach to induce muscular shortening involves folding the rectus muscle and suturing it directly to adjacent muscle tissue. However, this technique has gradually fallen out of favor due to its suboptimal long-term outcomes. The technique of antagonist muscle resection can be effectively employed in conjunction with agonist muscle recession. For instance, the recession of the lateral rectus muscle in the left eye will decrease the eyeball's leftward rotation. In contrast, the resection of the medial rectus muscle in the left eye will similarly reduce the extent of leftward rotation of the eyeball. The superior rectus muscle is also amenable to muscle excision [14-18].
Healing process and complications post-surgery for strabismus
Strabismus surgery has the potential to result in damage and initiate a sequence of wound-healing stages. The development of fibrous tissue and the manifestation of limitations are frequently observed following strabismus surgery and are deemed within the expected range if they do not surpass a certain threshold. Nevertheless, certain intricate treatments may lead to the occurrence of fibroproliferation and excessive scarring during the process of wound healing. These complications might involve many anatomical structures, including muscle, Tenon's capsule, conjunctiva, sclera, and orbital adipose tissue. To prevent the occurrence of this process, antifibrotic drugs and antimetabolites have been administered to avoid issues such as postoperative adhesions in strabismus, which can result in malfunction of motility. An atypical or heightened inflammatory process is accompanied by excessive extracellular matrix production, leading to fibrovascular tissue growth and excessive scar tissue. Postoperative adhesions are a significant factor contributing to the need for reoperation. However, the efficacy of adhesiolysis therapy and repositioning of the extraocular muscles is limited due to the challenges involved and the potential for forming new adhesions following reoperation [19-21].
Coagulation and hemostasis phase
The coagulation and hemostasis phase characterizes the initial stage of the wound healing process. The phrase above serves the purpose of preserving the circulatory system's functionality, ensuring the uninterrupted operation of essential organs in the event of an accident. In addition to its other tasks, this phase provides a matrix for the proliferation of cells necessary for wound healing. The process of hemostasis encompasses the participation of endothelial cells, platelets, the coagulation cascade, and the fibrinolysis pathway. These components collectively contribute to the formation of fibrin within the wound, ultimately facilitating the process of wound healing. In trauma, the microvasculature sustains damage, leading to the blood leakage into the wound. Smooth muscle contraction in blood vessels is a reflexive response. This measure is implemented to mitigate the occurrence of further hemorrhaging. This phenomenon exhibits a transient efficacy. The prolonged duration of this phenomenon can lead to hypoxia and acidosis within the wound, resulting in the subsequent relaxation of vascular smooth muscle and the reoccurrence of bleeding [22,23].
Besides the hemostasis process, the coagulation cascade is initiated via extrinsic and intrinsic mechanisms. These pathways serve to promote platelet aggregation and the creation of blood clots, hence mitigating excessive blood loss. When blood is extravasated into a wound, the components and platelets come into contact with collagen and other components of the extracellular matrix. Activating platelets leads to the synthesis of clotting factors and the subsequent development of blood clots. These clots are composed of fibronectin, fibrin, vitronectin, and thrombospondin. The process of blood clotting additionally serves as a framework for cellular migration throughout the inflammatory phase. The cytoplasm of platelets is composed of alpha granules that house a variety of cytokines and growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF-β), epidermal growth factor, and insulin-like growth factor. This molecule promotes the wound-healing cascade by initiating and recruiting neutrophils, endothelial cells, fibroblasts, and macrophages. Platelets also possess vasoactive amines, such as serotonin, which induce vasodilation and enhance vascular permeability. Consequently, these processes lead to fluid extravasation, contributing to edema development [22,23].
Inflammatory phase
The subsequent stage of wound healing is known as the inflammatory phase. This phase serves to enhance the body's immune response against microbes. This stage encompasses the activation of both humoral and cellular inflammatory mechanisms. The phase in question is categorized into two distinct stages, namely the early and late inflammatory phases. During the early phase of inflammation, the complement cascade is activated, and acute inflammatory cells, specifically polymorphonuclear cells such as neutrophils, eosinophils, and basophils, infiltrate the wound site. The objective of this intervention is to mitigate the occurrence of infection. Polymorphonuclear cells engage in the process of phagocytosis to eliminate invading germs. The migration of polymorphonuclear cells to the wound site typically initiates within 24 to 36 hours following injury. Several pro-inflammatory cytokines accompany this migration, including TNF-α, IL-1, IL-6, IL-11, IL-8, and other chemokines. Subsequently, the polymorphonuclear cells adhere to the endothelial cells in the venules around the wound site. Later, the polymorphonuclear cells undergo migration along the endothelial lining. Subsequently, endothelial cells initiate the production of chemokines to stimulate a more robust adhesion mechanism. Furthermore, polymorphonuclear cells exhibit the capability to eliminate bacteria and alien entities through the synthesis of proteolytic enzymes and the generation of free radical molecules [22,23].
The sluggish inflammatory phase manifests during a time frame of 48 to 72 hours after the occurrence of an injury. During this stage, polymorphonuclear cells are substituted by chronic inflammatory cells, specifically mononuclear cells and macrophages. The recruitment of mononuclear cells, and also macrophages is initiated by the presence of chemoattractive substances, including clotting factors, complement components, cytokines such as TNF-α, IL-1, PDGF, TGF-β, leukotriene B, and platelet factor IV. Mononuclear cells and macrophages are crucial contributors to cellular control and the provision of tissue growth factors, notably TGF-β fibroblast growth factor (FGF), which activates keratinocytes, fibroblasts, and endothelial cells. The absence of macrophages can result in suboptimal wound healing mechanisms characterized by impaired wound debridement, delayed proliferation and maturation of fibroblasts, and delayed angiogenesis, leading to insufficient fibrosis [22,23].
Proliferative phase
The primary stage of the wound healing process encompasses the hemostasis mechanism, which involves the body's immunological response aimed at averting potential infections. After attaining this milestone, the subsequent phase of wound healing is dedicated to restoring tissue integrity. The onset of the proliferative phase occurs on the third day following the damage and persists for two weeks. This process includes the migration of fibroblasts and the subsequent deposition of an extracellular matrix composed of fibrin and fibronectin. The presence of a substantial quantity of granulation tissue within the wound serves as evidence of this process. The initial event that occurs during the proliferative phase is the migration of fibroblasts. Fibroblasts are recruited in response to the presence of TGF-β and PDGF, both secreted by inflammatory cells and platelets. Migration of fibroblasts initiates on the third day after the occurrence of damage. Upon reaching the injury site, fibroblasts will increase and generate several matrix proteins, including hyaluronan, fibronectin, proteoglycan, and procollagen. The deposition of the extracellular matrix occurs within the initial week. Fibroblasts undergo a process of differentiation, ultimately giving rise to myofibroblasts. The latter has a distinct cytoskeletal structure characterized by strong actin filaments, facilitating pseudopodia formation. These pseudopodia enable myofibroblasts to establish connections with fibronectin and collagen molecules within the extracellular matrix. Wound contraction is crucial in facilitating the healing process by promoting the approximation of wound edges [22,23].
Collagen is a crucial component in the wound-healing process. Fibroblasts synthesize collagen and serve to maintain tissue integrity and strength while also contributing to the proliferative and remodeling stages of wound healing. Collagen serves as the fundamental basis for the creation of the intracellular matrix. Angiogenesis, the process of neovascularization, is known to contribute to the physiological mechanism of wound healing. Angiogenic factors are synthesized during the hemostasis phase. Endothelial cells exhibit a response to many angiogenic factors, including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), angiogenin, transforming growth factor-beta (TGF-β), and transforming growth factor-alpha (TGF-α). In addition to the presence of angiogenic factors, it is noteworthy that there exist several inhibitory factors, including steroids and angiostatin. These factors promote endothelial cell proliferation, inducing mitotic activation and stimulating cells to release endothelial growth factors. Under hypoxic conditions, several substances are released to initiate the proliferation and development of endothelial cells. Subsequently, endothelial cells release proteases serving the purpose of breaking down the basal lamina. Multiple biological activities are observed, including chemotaxis, proliferation, differentiation, and remodeling. Various factors, including endothelial cell growth factor, transforming growth factor-alpha (TGF-α), vascular endothelial growth factor (VEGF), fibrin, and lipid growth factors, contribute to neovascularization and blood vessel repair [22,23].
Remodeling phase
The remodeling phase is the concluding stage of the wound healing process, where the primary objective is to facilitate the development of fresh epithelial tissue and the subsequent formation of scar tissue. The initiation of the extracellular matrix synthesis commences with the development of granulation tissue. This particular phase has the potential to endure for a duration of one to two years or even an extended period [22] (Figure 1).
The remodeling process necessitates a harmonious interplay between the synthesis and degradation mechanisms. During this particular biological process, there is a progression in the maturation of the intracellular matrix, an increase in collagen levels, and the degradation of hyaluronic acid and fibronectin. The physiological mechanisms involved in collagen synthesis, degradation, and extracellular matrix remodeling persist for three weeks following an injury. Matrix metalloproteinase (MMP) enzymes play a pivotal role in collagen breakdown. The production of this enzyme is observed in neutrophils, macrophages, and fibroblasts. Tissue inhibitors modulate the activity of matrix metalloproteinases. As the level of these inhibitors intensifies, the function of matrix metalloproteinases diminishes, leading to the formation of a fresh matrix. The process of collagen creation becomes increasingly regular and is ultimately accomplished during the concluding stage of the remodeling phase. The underlying connective tissue undergoes contraction, resulting in the approximation of the wound edges. This phenomenon arises due to the interplay between fibroblasts and the extracellular matrix. Many substances, including FGF, TGF-β, and PDGF, facilitate this process. As the progression of the process unfolds, and the ongoing healing process ensues, there is a decline in the population of fibroblasts and macrophages, which can be attributed to the occurrence of apoptosis. Subsequently, the proliferation of capillaries, circulation of blood, and metabolic processes at the site of injury experience a decrease. The ultimate outcome of this stage is scar tissue formation characterized by robust compressive pressures and a diminished presence of cells and blood vessels [22,23].
TNF-α and neutrophils
Tumor necrosis factor-alpha (TNF-α) is a cytokine with pro-inflammatory properties known to manifest early throughout the inflammatory cascade. TNF-α belongs to a diverse group of cytokines known for their pro-inflammatory properties. These cytokines are extensively synthesized by macrophages and other cells associated with innate immunity, including Antigen antigen-presenting cells (APCs). These cells play a.
Crucial role in the intricate chemotactic mechanism of immune activation. The concept of adaptivity refers to the ability of a system or organism to adjust and respond. TNF-α inhibition has been beneficial in several inflammatory ocular conditions, including Behçet's disease, diffuse subretinal fibrosis syndrome, posterior scleritis, and retinal vascular tumors [24,25].
The genesis and development of inflammation need interactions among cytokine family members, wherein each type elicits a distinct response. In the present environment, TNF-α is typically synthesized with interleukins that can induce pro-inflammatory alterations in both the vascular and cellular domains. Hence, it is common for TNF-α and IL-1 to be co-synthesized promptly in response to bacterial infections [25].
TNF-α is a multifunctional cytokine involved in the inflammatory response. It serves as a key mediator in activating polymorphonuclear leukocytes (PMNs), initiating a cascade of events that ultimately lead to cellular death. In the interim, neutrophils, or PMN, constitute the predominant leukocyte population in the bloodstream, comprising approximately 4000-10,000/μL or 60-70% of the overall peripheral blood leukocyte count in adult individuals. The life cycle of neutrophils can be categorized into three distinct stages: bone marrow, blood, and tissue. In reaction to an infection, there is a rapid escalation in the generation of neutrophils from the bone marrow, resulting in an elevation in their count to 20,000/μL in the bloodstream. The production of neutrophils is induced by cytokines, specifically colony-stimulating factors (CSFs), which are released by various cell types in response to infection. These CSFs target stem cells in the bone marrow, promoting the proliferation and maturation of neutrophil precursors, including myeloblasts, promyelocytes, myelocytes, metamyelocytes, and ultimately, neutrophils [24] (Figure 2).
Neutrophils are the initial cells that respond promptly to many infections, particularly those caused by bacteria and fungi. Furthermore, these cells play a prominent role in maintaining homeostasis and are the primary cells involved in acute inflammation. The process of phagocytosis, wherein neutrophils and monocytes in the bloodstream engulf and eliminate germs, is characterized by a rapid sequence of events, including chemotaxis, phagocytosis, degranulation, respiratory burst, and the production of oxidants for bacterial eradication. Chemotaxis refers to the directed movement of neutrophils toward the site of infection, prompted by a favorable chemical gradient established by the chemoattractant chemotaxis. This chemotractant, such as the cytokine IL-8, is produced at the site of infection. During the inflammatory phase, neutrophils undergo an up-regulation of many factors, including TNF-α, macrophages, T cells, IL-8, IL-1, and IL-6. The quantification of inflammatory cell infiltration, namely PMN leukocytes, was performed using a light microscope on tissue slices. This quantification was done in each field of view, utilizing a binocular microscope with a 40x objective magnification and 10x ocular magnification [26]. The preparation was initially assessed with a magnification of 10x to observe the regions exhibiting the highest concentration of inflammatory cells. Subsequently, the neutrophil count was conducted with a magnification of 400x in three contiguous regions. The mean value of PMN was computed and later classified into distinct categories. The light category consisted of 1-50 cells, the moderate category consisted of 51-100 cells, the heavy category consisted of 101-200 cells, and the extremely heavy category comprised more than 201 cells [26,27].
Centella asiatica and asiatic acid
In Indonesia, the plant Centella asiatica is widely recognized by its vernacular name, Gotu Kola. Centella asiatica is classified under the Umbelliferae family. The gotu kola plant has been extensively utilized as a traditional medicinal remedy and thrives in regions characterized by tropical temperatures, including Indonesia, China, and India. Gotu Kola is recognized for its various advantageous properties, including antipyretic, diuretic, antibacterial, antiviral, and cognitive enhancement effects. Extensive research has been conducted on the advantages associated with the utilization of Centella asiatica, leading to a notable surge in its adoption throughout both Eastern and Western nations. Centella asiatica is known to contain many active chemicals, such as asiaticoside, thermolinic acid, madecassid acid, asiatic acid, batulinic acid, and madasiatic acid. The utilization of Asiatic acid in this study has demonstrated promising potential in several biological activities, including but not limited to anticancer, antidiabetic, anti-inflammatory, wound healing, antioxidant, hepatoprotective, and neuroprotective effects [9,28].
Asiatic acid has been found to possess several biological activities, including anti-inflammatory, anti-oxidant, anti-tumor, and wound healing properties. Asiatic acid has been found to exhibit advantageous effects on activating many enzymes and receptors, including PPAR-gamma and GABA receptors. Furthermore, it can block several receptors, including angiotensin receptors, endothelin 1 receptors, and toll-like receptors. Asiatic acid has been found to exhibit inhibitory effects on many enzymes, including alpha-glucosidase, C4 leukotriene synthase, and the ability to induce the MMP creation, collagen synthesis 1, plasminogen 1, and acetylcholine synthesis. Asiatic acid has been observed to exhibit antioxidant properties and demonstrate potential advantages in mitigating reactive oxidative species (ROS) impacts. In addition, Asiatic acid exhibits anti-inflammatory properties. It can modulate proinflammatory cytokines, slowing the progression of immunological disorders. Asiatic acid exhibits inhibitory properties towards NF-κB. Asiatic acid can inhibit the elevation of IκB-α phosphorylation induced by TNF-α. The findings of this study indicate that Asiatic acid shows a protective effect against the breakdown of the endothelium barrier. This protective effect may be attributed to the inhibition of NF-κB activation. Asiatic acid has demonstrated efficacy in suppressing the inflammatory response induced by inflammatory cytokines, including TNF-α, IL-6, and IL-8. Asiatic acid has been observed to exhibit advantageous effects regarding its anticomplement action, specifically by inhibiting hemolytic activity directed towards erythrocytes. Asiatic acid has been found to exhibit inhibitory effects on toll-like receptors, which are responsible for the activation of pro-inflammatory cytokines [9,29].
Asiatic acid exhibits diverse pharmacological properties at the molecular level, as evidenced by in vitro, in vivo, and in silico investigations. Asiatic acid can modulate molecular targets by altering gene expression and signaling cascades. Asiatic acid exerts regulatory control over the expression of many cytokines, including TNF-α, IL-1, IL-4, IL-5, IL-10, and IL-6. Moreover, it influences the expression of chemokines, growth factors, signaling molecules, adhesion molecules, apoptosis proteins, cell cycle proteins, genes, and receptors. Furthermore, Asiatic acid exerts regulatory effects on many transcription factors and their associated signaling pathways [9].
Asiatic acid has been found to exhibit advantageous effects in metabolic illnesses, including anti-diabetic, anti-hyperlipidemic, and anti-obesity properties. Its efficacy in the settings above is demonstrated through its inhibitory effects on alpha-glucosidase, glycogen phosphorylation, HMG co-reductase, and lipase enzyme activity. Asiatic acid exhibits neuroprotective properties that are advantageous in several situations, including Alzheimer's disease, dopamine-induced neurotoxicity, memory impairment associated with dementia, and epilepsy. The involved mechanisms encompass the suppression of beta-amyloid protein, modification of PAPRP, augmentation of acetylcholine synthesis, and activation of the glutamine synthetase enzyme. Asiatic acid has demonstrated certain advantages in cancer development, particularly in stomach cancer. The pathways implicated in this study encompass the suppression of nitric oxide synthase (NOS), cyclooxygenase-2 (COX-2), poly (ADP-ribose) polymerase (PARP), matrix metalloproteinase-2 (MMP-2), and MMP-9 [9].
Conclusion
The impact of Asiatic acid on the expression of TNF-α and the number of neutrophil cells in conjunctival tissue, tendons, and extraocular muscles following strabismus surgery is theoritically evident. The levels of TNF-α expression and the number of neutrophil cells are expected to decrease upon the administration of Asiatic acid compared to dexamethasone.
Acknowledgments
The authors would like to acknowledge all the staff and lecturer who assisted us during the writing of this Literature review.
Authors’ contributions
FA wrote the manuscript, RP & IW made a revision for the manuscript
Conflict of interest.
The authors declare no conflict of interest.
Funding
None.
Orcid:
Farahdila Adline: https://orcid.org/0009-0002-1726-6588
Reni Prastyani: https://www.orcid.org/0000-0002-8507-1689
Indri Wahyuni: https://orcid.org/0000-0003-3492-1601
---------------------------------------------------------------------------------
How to cite this article: Farahdila Adline, Reni Prastyani*, Indri Wahyuni, Potential therapy of asiatic acid to prevent scar remodeling post-strabismus surgery. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(2), 183-194. Link: http://jmpcr.samipubco.com/article_183798.html
---------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
