Document Type : Original Research Article
Authors
1 Research Scholar, KIIT School of Management, KIIT Deemed to be University, Bhubaneswar, India
2 Associate Professor, KIIT School of Management, KIIT Deemed to be University, Bhubaneswar, India
3 Senior Professor, KIIT School of Management, KIIT Deemed to be University, Bhubaneswar, India
Abstract
In recent years, marketing of live freshwater fish has assumed great importance in the state of Odisha. This process involves fish transportation in suitable containers (1000 litres capacity) from the fish pond to the fish market in live condition for sale. This article seeks to describe the various technical and commercial aspects of marketing of live fish, including its profitability in the state of Odisha. The profitability of a business or private firm refers to its potential to earn profits through its operations. Live fish is sold in several markets of Odisha, namely Bhubaneswar, Balasore, Baripada, etc. Further, this article tries to identify the factors responsible for successful transportation of live fish. It also tries to identify the reasons for which fish farmers and fish traders do live fish marketing (LFM). The article makes an effort to find out the markets where live fish is sold within Odisha State. The fish is kept alive during transit from the pond to the market by continuous supply of oxygen to the water through various means of aeration. Hence, although the entire process is tedious, it is undertaken with the profit motive. At present, people in Odisha are increasingly interested in buying live fish in contrast to dead or iced fish owing to its freshness and taste. Consequently, live fish fetches a price which is 40-60 percent higher (per kg) than the price of dead or iced fish. Live fish marketing in Odisha is carried out by both the fish farmers (i.e. producers) and the traders (i.e. retailers).
Graphical Abstract
Keywords
Introduction
Live fish marketing mainly involves the transportation of fish after harvesting from the pond to the fish market in live condition for sale through the auctioneers. This process though tedious is carried out for the economic rewards it confers on the fish farmer or fish trader involved. Owing to the preference of people to buy live fish over dead or iced fish because of its freshness and taste,live fish fetches a price which is substantially higher than the price of dead or iced fish. This increment of price when calculated over 300-350 kg of fish makes for a significant amount and provides incentive to the farmer to undertake live fish marketing.
Live fish marketing comprises the following steps:
- Harvesting the required quantity of fish and keeping the fish in boxtype hapanets in the source pond.
- Loading the fish into plastic/polythene lined carrier of a truck filled with water from the source pond.
- Moving fish from a pond to a market by truck while continuously aerating the water containing fish to maintain dissolved oxygen levels.
- After reaching the market, the fish is removed from the water in small lots and is brought to the auctioneer’s platform. At the auctioneer, the lot is weighed and auctioned to various fish traders-mostly retailers. The retailers then take the fish to their respective locations, dress the fish and sell it to their customers.
Objectives of the study
(i) To identify the factors responsible for successful transportation of live fish.
(ii) To identify the reasons for which fish farmers/fish traders do live fish marketing.
(iii) To find out which are the markets where live fish gets sold in Odisha.
Literature review
The entire literature review has been organized on the following three aspects:
(i) Transportation of live fish.
(ii) Chemical procedures for water and fish treatment during transport of live fish.
(iii) Live food fish markets of the world.
The transportation of live fish
The carriage of live fish from the farm to the market by truck is an important element of live fish vending besides meticulous reaping, sustaining and lifting the fish [10].
Transportation of live fish is a composite procedure where there is an elaborate interplay amongst the fish, the environment, the technical equipment, and various human factors. A few measures pertaining to the transport supply chain are as follows: (1) The groundwork phase, (2) making the fish ready (3), getting the transport vessel ready, (4) the filling up phase (5), the carriage phase, (6) the unburdening phase (7), cleanse and sanitize [12].
The transportation of fish from pond to market by truck while uninterruptedly oxygenating the water containing fish to maintain dissolved oxygen levels is the most crucial component of the entire process, since it assures the survivorship of a high proportion of fish by the time the truck reaches the market. The closed system and the open system are the two main methods used to transport live fish. The closed system is a watertight container that contains all the elements needed for survival. A watertight plastic bag that is partially filled with water and oxygen is the most basic of them. The open system comprises of water-filled vessels where the necessities of life are continuously supplied from external sources. The plainest of these is a small tank with an aerator stone [1,2].
The main points and concepts accompanying fish transport are as follows:
Quality of fish
Since the length of travel is usually rather extended, several hours at least, it is vital to prepare the fish ready in advance. The fish must be fit and in good physical shape and should be stopped from having meals before being transported, to empty the stomach and digestive system and hence decrease the liberation of waste catabolic products that affect the quality of transport water [1,2].
Poor specimens ought to be removed from the batch, especially if the temperature during delivery is high [1].
Oxygen
If the trip takes a long time, more oxygen must be supplied because the fish use up all of it during transportation [3]. It is important to note that providing an appropriate amount of dissolved oxygen is the single most important factor in fish transportation. Still, a large amount of oxygen in a tank does not necessarily indicate that the fish are healthy.
The fish species, age, mass, temperature, stress, and definite amount of dissolved oxygen and presence of other gases, all affect oxygen consumption during transportation. Without proper oxygen supply, the fish can perish in the transit tank. For Indian Major Carps, Chinese Carps, common carps, and other cyprinids, the lowest proposed dissolved oxygen (DO) level is 4 mg/litre [9].
As was previously noted, fish's ability to utilise oxygen is influenced by their tolerance to stress, water temperature, pH, carbon dioxide concentrations, and metabolic products like ammonia [1].
Fish weight and water temperature are the key determinants of oxygen intake by fish in relation to oxygen metabolism during transport [1].
Significant mortality is seen when transporting live fish because of insufficient oxygen in the water (Jhingaran, 1975). Prior to determine the fish density particular to each species, the duration of the transport, and the amount of water required for live fish transportation, it is imperative to comprehend the oxygen consumption rate.
Understanding how much oxygen different types of carp fish require is necessary for live transportation. The verified average oxygen consumption has shown a clear decline in fish species from surface-dwelling through column-dwelling to bottom-dwelling. The following was the species-wise tendency: 1060 g of silver carp (58.2 mg of oxygen per fish hour); 680 g of Catla (48.0 mg of oxygen per fish hour); 602 g of Rohu (41.5% of oxygen per fish hour); 1095 g of Mrigal (39.19 mg of oxygen per hour); 425 g of silver barb (21.25 mg of oxygen per fish hour); and 795 g of common carp (11.75 mg of oxygen per fish hour).
Different species of carps have shown dissimilar quantities of oxygen consumption during carriage [20].
Feeding state and oxygen consumption
Freshly fed fish typically use oxygen at a rate that is significantly higher than that of starved fish because to increased physical activity and direct action-processing of food.
Research indicates that when fish were fed, their oxygen consumption was more than twice as high as when they were not fed, and this increased consumption persisted for a while after the meal. Fish should not be fed for a minimum of twenty-four hours prior to being placed in the tightly packed circumstances of transport containers. It appears that anabolic processes of growth or tissue replacement, which only take place when the fish's maintenance energy requirements are met, are responsible for the meal-like imitation of oxygen consumption in a well-fed fish [13].
PH, carbon dioxide and ammonia
The amount of fish carried and how long they are carried affect the quality of the water [1]. The pH of the water is crucial because it directly affects how much toxic ammonia and carbon dioxide are present in the water [1].
Fish are negatively affected by increased carbon dioxide accumulations, which can also act as a barrier to fish transport. Carbon dioxide causes transport water to become acidic as a result of fish and bacterial breathing [1]. This lessens the ability of fish blood to transmit oxygen while also reducing the quantity of unionised ammonia in the water. Fish may perish even though oxygen levels appear to be adequate if carbon dioxide levels are high. The proper supply of fresh air is crucial for transport units. Tight coverings or lids on the units can produce an increase in carbon dioxide levels, which will stress the fish. If there is enough airflow, water oxygenation will reduce dissolved carbon dioxide buildup. Ammonia levels in transport water increase as a result of bacterial action on the waste and breakdown of fish proteins. It is feasible to decrease the anabolic rate of fish, which in turn decreases ammonia generation, by lowering the water temperature and so reducing fish activity [1]. Only when a meal has been delayed long enough for the stomach and intestines to empty should fish be delivered. By doing so, the amount of ammonia produced by bacterial activity will be reduced.
Ammonia excretion
The circumstance regarding the availability of food for the fish determines the rate of ammonia excretion. Due to the crucial breakdown and synthesis of body protein, a hungry fish excretes ammonia more frequently. The pH and temperature determine the kind of dissolved ammonia, and only the unionised type (NH3) is extremely dangerous. [13].
While oxygenation can release CO2 and ammonia into the atmosphere, it is normally essential to replace the water after 4 hours, and then again after 5 to 6 hours [9].
Temperature
A crucial factor is the temperature of the water. The pH remains higher and the fish metabolism declines in colder water. [1].
Because the water temperature in a tropical region can fluctuate greatly during the day, carriage is best done at night or in the early morning when conditions are at their lowest. High temperatures cause fish to have an accelerated metabolism, which increases their demand of oxygen and results in a considerable production of toxic carbon dioxide and ammonia [9].
Density and activity of transported fish
The economics of transportation is impacted by the density of fish during transit. To match the quantity of fish, the amount of water transported must be as shallow as practical.
It is challenging to establish a precise ceiling for the ideal transport density, which varies greatly depending on the species, size, and developmental stage. at salmon species, a rough estimate states that a density of up to 90-100 kg/cubic metre may be tolerated at temperatures lower than 8 oC; if the temperature is higher than 8 oC, a density of up to 50 kg/cubic metre is advised.
The proportion of fish in the total volume of transport water is another way to represent density [3].
When it comes to fry, the ratio of the volume of fish transported to the transport water shouldn't be more than 1:3 [1].
Much R&D has been encouraged by the Indian government to help with the transporting of live fish. To help farmers sell their food in live condition and increase revenue, the ICAR-Central Institute on Post Harvest Engineering and Technology- developed a device for shipping live fish.
The Live Fish Carrier System (LFCS) is powered only by DC power sourced from four non-polluting lead acid batteries. Its entire carrying capacity is 500 kg, and it can operate for around 80 kilometres on a single charge [6].
Stress during live fish transportation
Stress is characterised as a state in which the dynamic balance, or homeostasis, of animal species is jeopardised or disturbed due to the effects of inherent or non-innate stimuli, which are generally referred to as stressors.
(i) Handling: Carriage and operating steps comprise multiple potential stressors such as catching, filling, carriage, unburdening, temperature differentials, water quality alterations and stocking.
(ii) Crowding: Most favourable densities at filling up and in carriage tanks should always be ensured irrespective of financial benefit or comfort.
(iii) When the water temperature rises above the ideal range, thermal stress arises, causing stress reactions that require energy and perhaps reducing individual survivability [7].
As was previously indicated, minimising the stress on the fish is the biggest challenge when transporting live fish. According to Francis-Floyd (2002), stress is the result of a number of unfavourable conditions negatively impacting an animal's ability to maintain a normal physiological state. Fish can experience stress due to biological, chemical, or physical factors. [15].
The following are the actions to be taken to lessen transportation-related stress:
Sustentation of water quality is the core of stress curtailment in live fish transportation.
At the moment, farmers primarily take the following steps to manage pH: (i) add more pure oxygen; (ii) eliminate or nullify ammonia, (iii) add buffers to the water, (iv) abstain from food prior to transportation, (v) add salt or a mixture of sea water to reach isosmotic salinity, (vi) reduce temperature, (vii) use anaesthetics, and (viii) add probiotics [21].
Small amounts of oxygen or low water quality due to inadequate water replacement, which results in a build-up of expelled carbon dioxide and ammonia, can potentially cause stress throughout the conveyance [24].
Live fish transportation and welfare of fish
Transporting live fish is a common practise in aquaculture, although it can have negative effects on the wellbeing of the fish. In order to grow or sell, live fish are frequently transported between farms or to marketplaces. Typical annoyances associated with live transportation include improper handling, exposure to the air, lack of food, poor water quality, inappropriate transit densities, sudden changes in water temperature, etc. Maintaining the health and welfare of live fish during transportation is a challenging task. Fish that have been transported exhibit physical signs of stress, and it is well recognised that severe stress lowers fish energy and increases fish mortality [8].
The fish farming industry cannot allow to disregard fish welfare when negative propaganda about it could affect sales substantially. The important spheres where welfare issues have existence at present are as follows: too high a stocking density, feeding procedures which do not get food to all of the fish, lack of success in knocking fish unconscious, pain in various circumstances, lack of adequate environmental arousal, disease control, transportation of live fish, etc. [17].
To reiterate, the welfare issues for farmed fish include the following:
(i) With as little food deprivation period before catching, transporting, and killing as possible, fish are given sufficient nourishment according to species and age. (ii) An environment that is favourable for that species, with favourable levels of flowrate, light intensity, dissolved oxygen, ammonia, acidity, amount of organic matter, and other factors. (iii) Fostering environments with low risk of injury, avoiding harsh methods of capture and handling, and offering disease prevention (iv) Nurturing conditions in which fish do not experience fear and chronic stress, such as shielding fish from predators, treating fish with care while being captured and handled, and employing compassionate methods of slaughter (Relic et al., 2015) [16]. (iv) Nurturing conditions in which fish can exhibit normal species-specific behaviour, such as providing enough space for swimming.
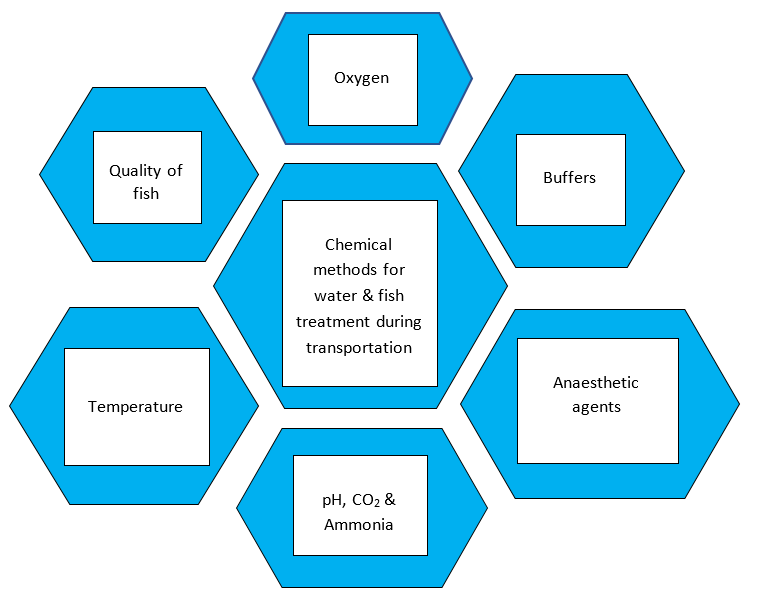
(ii) Chemical techniques for treating water and fish during live fish transport:
An important aspect of the overall problem of fish transportation is the use of chemical ways to deal with the transport medium to increase the capacity volume of transport units and prevent harm to the fish's physiology and health. These techniques include the use of anaesthetics, compounds that produce oxygen and harden water, bacteriostatic, buffering, and antifoam agents, among other things.
Use of fish sedatives
The fish should be sedated to limit oxygen use, carbon dioxide production, and ammonia production during transport. Deep sedation is undesirable, though, as the fish risk sinking, clumping together, and succumbing.
Always give careful consideration to a chemical's legal position and any potential effects on the customer. Among the wide gamut of sedatives, the following appear to be utilized most regularly:
- chemical anaesthetic agents
Triclone methane sulfonate (MS-222), benzocaine (etilparaaminobenzoate), 2-phenoxyethanol, etomidate, and carbon dioxide are the most often used synthetic anaesthetics (Ross & Ross, 2008; Weber et al., 2009) [4].
- Tricainemethanesulfonate(MS-222):
The MS-222 substance, which is found in a white, clear, and colourless talc, has a basic ethyl 3- aminobenzoate molecule. The goal of this composite has been stated by numerous studies as serving as fish anaesthesia (Zahl et al., 2012; Ghanawi et al., 2013) [4]. MS-222 is a very gentle tranquiliser and fish effortlessly recuperate from its repercussions even after a long condition of oblivion.
Use and dosage of MS-222
Tricaine methane sulphonate is used in aquaculture for regular course of actions such as selecting fish, classifying, carriage, and blood representative selection (Popovic et al., 2012).
Commonly, prescription of MS-222 extends over 25 to 250 mg/litre in a water soak. Subjections greater than 10 minutes at >50 mg/litre may give rise to loss of life (Marking, 1967) [29].
- Quinaldine (2-4methylchinolin)
Because it contains poison, it needs to be handled carefully. When the fish are transported in a large volume of water, such a large tank, they are typically served with it [1].
- phenoxyethanol
It is a clear, colourless, barely odourless fluid that dissolves readily in water. It is used as a well-liked anaesthetic, bactericide, and fungicide that is beneficial for abdominal surgery or surgical incisions at a reasonably low cost. (Ross & Ross, 2008; Brown, 2011; Aqui-S, 2013) [4].
Use and dosage of phenoxyethanol
Because of its immediate effects and short recovery period, phenoloxyethanol is frequently employed in aquaculture, according to Javadi Moosavi et al. (2014) (Shaluei et al. 2012; Javadi Moosavi et al. 2014). Because of its accessibility, reliability, and effectiveness, it is widely used in the USA and the EU for research on aquarium fish and aquaculture (Coyle et al., 2004; Ogretmen and Gokcik, 2013), as well as to sedate aquarium fish and non-food fish during transportation (Brown, 2011). (Weber et al., 2009; Ucar and Atamanalap, 2010) [29].
Phenoxyethanol's effective anaesthetic doses range from 0.06 to 1.2 ml/litre for a variety of fish species. At doses between 0.1 and 0.6 ml/litre, it has a broad margin of safety and has been documented to produce a range of effects, from mild drowsiness to surgical anaesthetic.
- Benzocaine compounds
This substance is colourless, flavourless, and odourless, and it resembles MS-222. The dosage is very similar to that of MS-222.Compared to other readily available anaesthetic chemicals, this one is less expensive (Gomes, Chippari-Gomes, Lopes, Roubach, and Araujo-Lima, 2011). [4].
- etomidate
The anaesthetic etomidate, which dissolves in water, has been widely used by fish experimenters. As opposed to employing MS-222 anaesthesia, anaesthesia duration was completed in 1-2 minutes without aberrant activity, resulting in a quicker recovery (Aqui-s, 2013; Ross & Ross, 2008) [4].
- natural anesthetic agents
- Terrestrial resources:
Researchers have claimed that specific earthbound flora contain anaesthetic substances. Clove oil (Eugenia aromaticum), according to some researchers, is the most effective natural substance used for fish anaesthesia (Aydin et al., 2015; Fernandos, Bastos, Barreto, Lourenco & Penhab, 2017) [4].
Clove oil is a dark brown liquid that is obtained by purifying the leaves, stem, and flowers of the clove plant Syzygium aromaticum (Eugenia caryophyllata). Eugenol (4-allyl-2-methoxyphenol, C10H12O2, molar weight: 164.2 g/ltr) and iso-eugenol (4-propenyl-2-methoxyphenol), which together make up 90-95% of the weight of the oil, are the functional components of clove oil.
Clove oil use and dosage
Clove oil has been studied as a possible sedative for aquarium fish and other types of artificially bred fish (Holloway et al., 2004; Velisek et al., 2005 a,b, 2006). It is an interesting anaesthetic because, as noted by Griffiths (2000), Cho and Heath (2000), and Mylonas et al. (2005), it is reasonably priced and safe for both people and the environment. Doses of 2.0 to 150 mg/litre of clove oil are indicated for anaesthesia [29]. Clove oil is prescribed for anaesthesia in doses ranging from 2.0 to 150 mg/litre [29]. Clove oil prescriptions differed from 2.5 to 50 mg/litre for hybrid striped bass (Morone chrysops x M saxatilis) (Davis and Griffin,2004), 5-9 mg/litre for largemouth bass micropterus salmoides (Cooke et al., 2004) and between 100 (Small, 2003) and 300 mg/litre(Waterstrat,1999) for channel catfish Ictalurus punctatus [14].
Clove oil has been widely utilized in multiple fish type, and the outcomes demonstrate that the material is a cost-effective substitute for the chemicals utilized in fish anaesthesia(Ross & Ross, 2008) [18].
- aquatic resources: seaweed
Seaweed has a variety of bioactivities and uses, making it a promising natural medium.
Alkaloids, flavonoids, phenolics, steroids, tannins, and saponins are just a few of the functional composites found in E. cottonii seaweed. These compounds are sedatives or soporifics and are toxic to fish (Harborne, 1987; Rahayu, 2015; Rohyani, Aryanti & Suripto, 2015).E. Cottonii is eligible to be used as an anaesthetic because of the presence of these substances [4].
Application of sodium chloride and calcium chloride
By incorporating calcium and salt chlorides into the carrier water, fish operating stress and delayed death can be minimised. The calcium ion lessens salt and water equilibrium and metabolic problems, whereas the sodium ion tends to stiffen the fish and diminish mucus production [1].
Salt loss from the circulation is accelerated by carrying stress and mucus loss, increasing the fish's energy requirements. Tetany (nerve and muscular spasms) and heart failure can both be brought on by uncontrolled salt loss. NaCl can be used to help freshwater fish feel less stressed by acting as an osmoregulatory tool (Burgdorf-Moisuk et al., 2011; Camargo et al., 2006) [27].
Chemicals as oxygen sources:
Regarding the use of synthetic materials as oxygen sources during fish transportation, there are differing views. Using transported carp fry as test subjects, Huilgol and Patil (1975) found that adding one drop of hydrogen peroxide (6 percent concentration) to one litre of water increased the oxygen content by 1.5 mg/litre at 240 C [1].
Nearly everywhere in the world, hydrogen peroxide is readily available as a medicinal mixture. As heat evolves, hydrogen peroxide decomposes into water and gaseous oxygen. In general, natural disintegration occurs slowly, although it can be accelerated by light or a broad range of components and composites (Schumb, 1955).
The poisonous character of hydrogen peroxide indicates that if it is to be utilized as a wellspring of dissolved oxygen for fish transport, it must be utilized as a highly watered down mixture [19].
Bacteriostatic chemicals
Anti-bacterials are also used in transport units to prevent bacterial growth [1].
Anti-bacterials are medicines of non-artificial or chemical origin that have the power to eliminate or to restrain the ballooning of microbes. Penicilins, cephalosprins, carbapenems, streptomycin, neomycin, erythromycin, etc. are some instances of antibacterial medicines that are utilized in fish farms [28].
However, particular stress should be given to restricting utilization of antibacterial drugs and residue contamination in the fishing sector because of its potential dangers to public well-being and fighting antimicrobial resistance (AMR) for safe aquatic food production [11].
In addition to the above, utilizing iodine as a bactericide during fish carriage during aquaculture operations has a favourable role in sustaining a safeguarding level of resistant position and helping in bacterial disease resistance [22].
Buffers
Buffers like tris-buffer (tris hydroxyl methyl amino methane), among other synthetic supplements, are useful in maintaining pH at a desirable value of 7 to 8 [1].
Ammonia control
It is advised to utilise clinoptilolite, a zeolite mineral, to control ammonia collection in the transport bags when it is predicted that the carriage would be extended [1].
Ammonia is the crucial final product in the disintegration of proteins in fish. Total ammonia nitrogen (TAN) consists of toxic (unionized) ammonia NH3 and non-toxic(ionized) ammonia NH4+. Only a small part of the TAN exists as toxic (unionized) ammonia,and an equilibrium exists between it and the nontoxic ionized ammonia:
NH3 + H+ = NH4+
The percentage of TAN in the toxic form escalates as the temperature and pH of the water magnify [25].
Two methods are usually used to control the collection of ammonia in transport water: blocking ammonia generation by modifying the catabolism of the fishes, and eliminating ammonia from the water after it has been excreted. Some suggested methods of reducing the catabolic rates (and hence the rate of ammonia excretion of confined fishes) are decreasing the water temperature (Philips and Brockway, 1954; Voss, 1979), adding anaesthetics to the water (Philips and Brockway, 1954; Nemoto, 1957; Mc Farland, 1960; Smit et al., 1977) and holding back food for a drawn out period before the fish are transported (Philips and Brockway, 1954;Van de Sande, 1974).
A variety of ion-exchange organic substances have the capacity to eliminate ammonia from fresh water. Clinoptilolite is a naturally occurring zeolite with a typical unit-cell formula of Na4K4Al8Si40O96.24H2O [26].
Antifoam chemicals
When pharmaceuticals are used to transport fish or on water that is significantly contaminated with organic waste (discharges and defecations, such as mucus and excrements), the formation of froth and rubbish can be extremely bothersome. Foam obscures the fish's movement and prevents oxygen from reaching to the water's surface [1].
(iii) Live food fish markets of the world:
The biggest markets for live fin fish products are frequently those with relatively wealthy populations, which include sizable numbers of Chinese citizens.
(i) Hong Kong:
Hong Kong is the principal destination for live fin fish imports from Australia. In 1995, 28,213 tonnes of live fin fish were imported into Hong Kong, the majority of which were marine species (Sudari, 1996). The leading exporters of live marine fin fish to Hong Kong include China, Taiwan, Thailand, the Philippines, Malaysia, and Indonesia (Sudari, 1996 [5]).
In the Hong Kong live marine fish trade, 3 sections can be identified:
- The indigenous home consumption market,
- The affordable restaurant market, and
- The expensive restaurant market.
There are remarkable differences in demand and therefore price in the Hong Kong live fish market right through the year (Li,1996; Sudari,1996).
Until the 1980s, live reef fish eaten in Hong Kong came mostly from local waters and the northern zone of the South China Sea (Johannes and Ripen, 1995). As coveted species became depleted locally and as market demand for magnitude and newness expanded, live fish were shipped or flown in from faraway places such as Indonesia, the Maldives, Australia and the Western Pacific. Imports of live fish increased from 2000 tonnes in the late 1980s to about 15000 tonnes by the mid-1990s. Hong Kong is the leading importer of live reef fish for food in South East Asia, comprising as much as 60% of the entire yearly regional commerce of 25000 tonnes (Johannes and Reipen,1995) [30].
(ii) Taiwan:
In 1994, Taiwan received 59 tonnes of live marine fish imports, the majority of which came from Thailand (Sudari, 1996). The grouper made up 83% of all imports.
Taiwanese customers favour live fish items more than Hong Kong consumers do, whereas oceanic species are less popular. Prices are often reduced as a result [5].
(iii) Singapore:
In 1994, imports of live marine fin fish into Singapore totaled 1841 tons, most (96%) have their origin from western Malaysia (Sudari,1996) [5].
(iv) Japan:
Despite having the largest market in the world for seafood products, Japan's market for live fish products is less than Hong Kong's (Sudari, 1996). According to Sudari (1996) [5], Japan imports about 5000 tonnes of live fish annually.
(v) Other markets:
Although the major markets for live fin fish are Hong Kong, Southern China, Taiwan, Singapore, and Japan, additional markets for live seafood products are developing in Asia.
Due to rapid economic growth and rising income, live seafood markets are expected to grow significantly in South Korea, Malaysia, Thailand, Indonesia, and the Philippines (Sudari, 1996 [5]).
Research gaps
As the researcher was searching for extant literature on live fish marketing in Odisha, he stumbled upon the following discovery. There was practically no literature available on live fish marketing in Odisha, although live fish gets sold in several markets of the state.
The conceptual framework
Based on the steps involved in live fish marketing, a conceptual framework has been developed as follows:
From the conceptual framework, it is clear that a high percentage of survival of fish at the market is very desirable, since more live fish at the market mean more money for the farmer and greater profits. During transportation, continuous aeration is essential, since more dissolved oxygen in the water mean less carbon dioxide and less ammonia and also less stress for the fish and better survival. Even after reaching the market, the water can be aerated by splashing with the aluminium vessel or by a recirculating diesel pump. Hence the one variable that ensures high survival and greater profitability is dissolved oxygen content of water and lack of stress to the fish.
Data and methodology
The study has used both primary and secondary data collected from various sources. Structured questionnaires were used to collect information from the wholesale and retail fish markets through key informant surveys. Two farmers(producers) and ten traders(retailers) were interviewed and asked to answer the questionnaire.
The secondary information was collected from officials of Directorate of Fisheries, Cuttack. The survey was conducted in a few fish markets of Odisha, namely Bhubaneswar, Balasore and Baripada which are noteworthy for live fish sales and marketing. Information was collected on prices, mode of transportation used from pond to market, the various species sold etc. As mentioned earlier, several fish traders were also interviewed in the course of the study.
Since the last few years, production of freshwater fish in Odisha is showing an upward trend. Hence a substantial percentage of the overall production can be subject to live fish marketing. Fish production is increasing in Odisha, as Table 1 on district-wise fish production proves. Statistical tools like mean, standard deviation, one sample t-test, ANOVA, Duncan’s Multiple Range Test, and multiple regression are used to study the trend in detail. The calculated t-value 4.860 has been found to be significant at 5% level (P<0.05) by taking the total production of the state on 2016-17 (393730 MT) as base. This significant t-value indicates the acceptable growth of freshwater fish production in the Odisha state over the period of five years i.e. from 2016-17 to 2020-21.
Table 2 presents the rate of growth of freshwater fish production over the period 2016-21. The regression coefficient (R2) 0.901 shows the good model fitting of annual fish production over the time. The rate of annual growth may be expressed mathematically as follow:
Production = 359102.00 + 45136.6 * Year (1)
where, year is the independent variable, and production is the dependent variable Table 3 and Figure 2 depict the percentile distribution of each zone in respect of total freshwater fish production. While central zone maintains the lead, the western and southern zones remain at middle and bottom respectively all through. While the contribution of central zone whirls around 40% of the total production of the state, the same oscillates around 32% and 27% for western and southern respectively in that regard.
The results of analysis of variance on total fish production for 2016-17 to 2020-21 in respect of different zones of Odisha (Table-4) give significant F-value (9.885) at 5% level (P<0.05). This shows there is significant difference in total fish production in different zone during this period.
Table 5 and Figure 3 presents the means and SDs of different revenue zones segregated from respective districts during this period. It is observed that central zone produces the highest freshwater fish and remains the top whereas southern zone produces least in every year. On the process, the central zone produces 202527.40 MT (SD 33507.95) freshwater fish annually whereas western and southern zone have mean annual figures as 159283.40 MT (SD 22025.63) and 132701.40 MT (SD 16641.23), respectively. The superscript “A” indicates the significant difference of central zone from the rest two similar zones (western and southern) with superscript “B” in respect of annual fish production. Hence, in consideration of quantity of fish production annually, central zone produces significantly more than other zones. Accordingly, people of central zone prefer culture as well as consumption of freshwater fish more than rest of Odisha.
Table 6 provides information on the cost of production that is incurred in producing 1 kg of fish in the context of Odisha:
As far as profitability of live fish is concerned, the live fish sells for Rs 170/kg at Baripada, Rs 150 /kg at Balasore and Rs. 200-220 /kg at Bhubaneswar, where iced fish fetches a price of Rs 100-130 /kg depending on size.
The increment in price for live fish is more than 50% per kg over iced fish of comparable size, which ensures a handsome profit for the farmer or trader involved in live fish marketing.
Results and discussion
Research Objective 1: To identify the factors responsible for successful transportation of live fish. By referring to the literature review, we can identify the factors with a bearing on the successful transportation of live fish. The main factors include Quality of fish, dissolved oxygen, water pH, carbon dioxide, ammonia, temperature, density of fish etc. The provision of a suitable amount of dissolved oxygen is the most crucial element in the transportation of live fish. Fish's capacity to utilise oxygen is influenced by their tolerance to stress, water temperature, pH, carbon dioxide concentrations, and metabolic products like ammonia. Research Objective 2: To identify the reasons for which fish farmers/fish traders do live fish marketing.Both fish farmers and fish traders carry out live fish marketing with the profit motive. As is clear from Table 3, the cost of production of 1 kg of fish in Odisha is Rs. 80 per kg. As is evident from a previous paragraph, live fish sells for Rs.150 /kg at the Balasore market and Rs. 170 /kg at the Baripada market, assuring a handsome profit for the farmer. Along with the fish farmers, several fish traders were also interviewed in the course of the study. These traders (mainly retailers) collected the fish from Pipili area (near Bhubaneswar) and sold the fish at the Bhubaneswar Unit-IV market and also at the Ravi Talkies fish market in Old Town. One of the traders Bharat Behera reported that he collected his fish from the Balipatana area and brought his fish live to the Ravi talkies market in a crate filled with water and mounted on his motorcycle. He mentioned that fish ponds were harvested in the Balipatana area and about 15-25 kgs of fish were loaded in a crate filled with the pond water. The water in the crate vibrated due to the motion of his motorcycle and received oxygen from the atmosphere. He sells the following species to his customers: Rohu (Labeo rohita), Catla (Catla catla), and Mrigal (Cirrhinus Mrigala). His selling price for Rohu is Rs.200 /kg now. His selling price for Catla is Rs.220 /kg and for Mrigal is also Rs.220 /kg.
Research Objective 3: To find out which are the markets where live fish gets sold in Odisha. The market places, where live fish gets sold in Odisha are mentioned in Table 7. The table provides a break-up of the districts and the market places in each district, where live fish gets sold within the state of Odisha. The main markets include Bhubaneswar (District-Khurda), Balasore town (District-Balasore), Baripada (District-Mayurbhanj), etc.
Conclusion
Live fish marketing in Odisha is a profitable operation. The selling price of live fish in Odisha is much higher than the cost of production. This differential between the selling price and the cost of production determines the profitability of live fish marketing. Both the fish farmers and fish traders are interested to engage in the business owing to its profitability. This is also the reason live fish gets sold in several markets of the state. Furthermore, the demand for live fish in Odisha is owing to its freshness and superior taste.
The other important aspect of live fish marketing is transportation of live fish from pond to market place in truck carriers filled with pond water, while continuously aerating the water to maintain dissolved oxygen levels in the water. Adequate dissolved oxygen in the transport water is the most crucial factor that keeps the fish alive until the truck reaches the market. The ability of fish to use oxygen is contingent on their tolerance to stress, water temperature, pH and concentrations of carbon dioxide and metabolic products such as ammonia.
Acknowledgements
The authors wish to thank and acknowledge the contributions from the fish farmers from Balasore, who sell live fish in Baripada and Balasore markets. They also wish to thank the fish traders (retailers) from Bhubaneswar, who were very forthcoming in providing relevant information about live fish. The authors also appreciate the support from the officials of Directorate of Fisheries, Cuttack and Central Institute of Freshwater Aquaculture (CIFA), Bhubaneswar who were very helpful in providing production and cost data on fresh water fish in Odisha. Without their active support and cooperation, it would not have been possible to complete this research paper.
Ethical issues
(If the manuscript is Original Article or Review, this field should be filled.) Central Institute of Freshwater Aquaculture (CIFA), Bhubaneswar, Odisha, India
Competing interests
Not Applicable
Authors’ contributions
The authors contributed in equal measure towards the manuscript with regard to selecting the criteria for farmers and traders, conceptual framework and methodology adopted for carrying out the research. All the authors participated in the data analysis, drafting and revision of the publication along with accepting responsibility for all components of this work.
Orcid:
Tapas Ranjan Das: https://www.orcid.org/0009-0009-7692-0800
Abhishek Kumar: https://www.orcid.org/0000-0002-0876-3219
Ipseeta Satpathy: https://www.orcid.org/0000-0002-0155-5548
Arvind Tripathy: https://www.orcid.org/0000-0001-9778-4128
--------------------------------------------------------------------------------
How to cite this article: Tapas Ranjan Das, Abhishek Kumar*, Ipseeta Satpathy, Arvind Tripathy, Maximizing profitability and freshness: Chemical treatment techniques for live fish transportation in Odisha. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(2), 195-212. Link: http://jmpcr.samipubco.com/article_183815.html
--------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
