Document Type : Original Research Article
Authors
- Galih Sampoerno 1
- Widya Saraswati 1
- Hasna Shabrina 2
- Nathania Nurani Fripertiwi 3
- Sarah Aniqoh Fauzya 3
1 Department of Conservative Dentistry, Faculty of Dentistry Airlangga University, Surabaya, Indonesia
2 onservative Dentistry Specialist Program, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia
3 Undergraduate Program, Faculty of Dentistry Airlangga University, Surabaya, Indonesia
Abstract
In dental pulp tissue, lipopolysaccharide invasion may be the cause of nerve cell death, which lowers the total amount of nerve cells in the pulp. The disease causes the pulp of teeth to deteriorate, which may have an impact on the risk of endodontic flare-ups. One sort of cell death used to lower the quantity of undesirable cells that might compromise pulp quality is called necroptosis. The goal of this study is to understand how Porphyromonas gingivalis lipopolysaccharide injection causes the necroptosis of dental pulp nerve cells. For experimental section, 32 Sprague Dawley rats in total were divided into 2 groups. Lower incisor access was induced in the treatment group using Porphyromonas gingivalis lipopolysaccharide, whereas in the control group, lower incisor access was performed and filled with GIC. A day later, both groups were closed. Next, preparation slides were made, and using immunohistochemical labeling, the number of nerve cells and Mixed Lineage Kinase Domain-Like Protein (MLKL) was measured. The findings demonstrate a substantial difference between the lipopolysaccharide treatment group and the control group, as well as an increase in MLKL expression and a decrease in nerve cell count following lipopolysaccharide injection. Finally, this study shows that the injection of Porphyromonas gingivalis lipopolysaccharide results in necroptosis in dental pulp tissue nerve cells.
Graphical Abstract
Keywords
Introduction
Dental cavities expose the pulp, opening a path for gram-negative anaerobic bacteria to produce endodontic infection, which leads to intraradicular infection, inflammation, and death of the pulp tissue [1]. Inflammation of the pulp tissue is caused by lipopolysaccharide (LPS), which is a product of anaerobic gram-negative bacteria, such as Porphyromonas gingivalis [2]. Inflamed pulp is associated with nerve changes in the pulp, which leads to pain, degenerative changes, and pulpal necrosis [3]. Hence, a proper endodontic procedure is required to treat the infection by performing root canal treatment [4]. In root canal treatment sessions, flare-ups can occur, which are pain and swelling affected by the pulp diagnosis [3]. The normal dental pulp that had been extracted showed a more noticeable flare-up reaction than the pulp that had been inflamed by LPS induction and then extracted [5]. An increase in the intensity of the impulse suggests that a high number of nerve fibers have been stimulated [6]. In pulps with irreversible pulpitis, moderate to severe neurodegenerative myelinated nerves occur, including granular degeneration, flaking degeneration, and disintegration of the myelin sheath and vacuolization [7].
According to several studies, nerve cell death caused by progressive inflammation due to lipopolysaccharide, which can be achieved in various ways, one of which by targeting Toll-like receptor 4 (TLR-4) and activating the MyD88 pathway, which is one of the mechanisms of the cell death pathway that includes necroptosis [8,9]. Tumor necrosis factor (TNF) and the other cell death receptors mediate necroptosis. Receptor Interacting Serine/Threonine Kinase 3 (RIPK3) is activated during receptor initiation, and Mixed lineage kinase domain-like protein (MLKL) is phosphorylated as a result [10]. In a related study, the pathway of cell death via necroptosis was identified using 30 LPS-induced mouse samples. Using immunohistochemistry, they looked at proteins implicated in necroptosis, such RIPK3 and MLKL, to determine whether these cells had necroptosis. After administering LPS for 24 hours, the research observed an increase in the expression of MLKL and RIPK3 [11]. The previous study reported elevated expression of RIPK3 and MLKL in male rats administered LPS from P. gingivalis bacteria, which were significant indications of necroptosis in the study [12]. Since the mechanism underlying the necroptosis pathway in dental pulp nerve cells is unknown, it is necessary to identify the MLKL expression as a particular protein associated with the mechanism of necroptosis produced by the injection of P. gingivalis lipopolysaccharide as observed after 24 hours. This study aimed to know the process of necroptosis of dental pulp nerve cells after administration of Porphyromonas gingivalis lipopolysaccharide.
Materials and methods
Research design
With 32 Sprague Dawley rats serving as the experimental animals, the study was conducted in an experimental laboratory setting with a post-test only control group. All described procedures had been evaluated and authorized by the Universitas Airlangga Faculty of Dental Medicine Health Research Ethical Clearance Commission (Certificate number 522/HRECC.FODM/VIII/2022). MLKL expression increased and the number of nerve cells decreased, indicating that LPS had an influence on the necroptosis of nerve cells in the pulp tissue.
Research method
This study involved 32 male Sprague Dawley rats with the inclusion criteria (good health, aged 20 weeks, weighing 425-450 grams body weight, and fully erupted mandibular incisors). Two groups of Sprague Dawley rats were randomly assigned: one for LPS treatment and the other for control. Prior to treatment, ketamine (80 mg/kg) and xylazine (10 mg/kg) were given intraperitoneally and diluted in sterile Phosphate Buffer Saline (PBS) to anesthetize all samples. Using a fissure bur, the mandibular incisors of each sample were severed at the level of interdental papillae (3 mm) to create a flat surface. The purpose of this step is to make it easier to use a spherical bur to puncture the pulp ceiling. Glass ionomer cement (GIC) was used to seal the pulp chamber after ten micropipettes of stock isolated from Porphyromonas gingivalis lipopolysaccharide (Ultrapure lipopolysaccharide from Porphyromonas gingivalis–TLR4 ligand, Catalog # tlrl-ppglps. Version #14F18-MM. Invivo-Gen.3950 Sorrento Valley Blvd. Suite 100 San Diego, CA 92121-USA). Termination and mandibular isolation were carried out a day later. The mandibles were turned into a paraffin block after being fixed for 24 hours in 10% buffered formaline and 30 days decalcified in 4% Ethylene Diamine Tetraacetic Acid (EDTA).
After slicing the paraffin blocks into 4 µm slices with a microtome, they were heated for a whole night at 56-58 ⃘C. The incision preparation was incubated in 3% hydrogen peroxide for 30 minutes at room temperature to eliminate endogenous peroxidic activity. The tissue incisions underwent four 5-minute xylol deparaffinization, five minutes of radiant alcohol rehydration (absolute, 95%, and 70% alcohol), and five minutes of running water washing. Hematoxylin Mayer was used as a counterstain after immunohistological staining with a monoclonal antibody, anti-MLKL (SantaCruz Biotech, USA). Under a light microscope (Nikon E100 LED binocular microscope with camera system Sony ILCE A-7. Nikon, NY, USA) with 1000x magnification in 20 distinct field analyses, the expression of MLKL and nerve cells in the pulp tissue were seen.
Data analysis
The Independent t-test was utilized to examine variations in the quantity of nerve cells and MLKL expression, while the Shapiro-Wilk test was employed to evaluate the distribution of the data (Figure 2). In addition, a Pearson Correlation test was run to ascertain the association between MLKL and the quantity of nerve cells. For all tests, IBM SPSS Statistics 24 (SPSS for Windows) was utilized.
Results
Necroptosis of nerve cells in dental pulp tissue
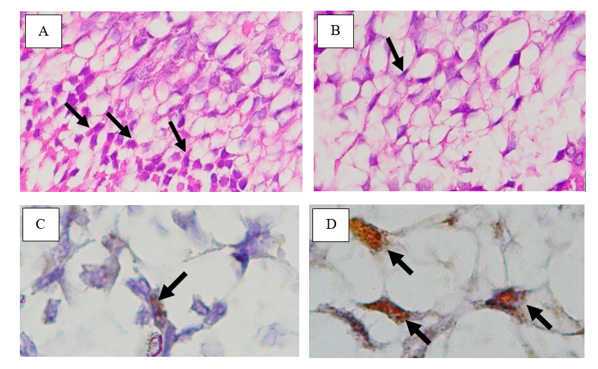
Histological observations of nerve cells in dental pulp tissue and MLKL expression are displayed in Figure 1. Histological examinations of the dental pulp nerve cells from cross-sections in the sagittal direction on mandibular incisors using Hematoxylin-Eosin staining revealed that the cells were star-shaped, polyhedral, or pyramidal, with red in the cytoplasm and blue in the nucleus. The histology of nerve cells in control and LPS treatment groups can be seen in Figure 1 (A-B), respectively. Histological examinations of MLKL expression using immunohistochemistry are indicated with brown color in the cell cytoplasm and are either star or polyhedral in shape. The MLKL expression of the control and LPS treatment groups can be seen in Figure 1 (C-D), respectively.
The results of the study on necroptosis after administration of lipopolysaccharide based on the expression of Mixed Lineage Kinase Domain-like Protein (MLKL) and the number of nerve cells in dental pulp tissue are pesented in Table 1.
Before testing the differences in MLKL expression and the number of nerve cells between treatment groups, each group was tested for data distribution using the Shapiro-Wilk test to see whether the data to be analyzed were normally distributed. The normal distribution test results are summarized in Table 2.
To find out the differences in MLKL expression and the number of nerve cells between treatment groups, an Independent t-test was performed. The test results differed significantly between groups (p = 0.001). The Independent t-test results are provided in Table 3.
To determine the relationship between MLKL expression and the number of nerve cells, the Pearson Correlation test was performed. The test results showed a negative relationship (r = -0.474) between MLKL expression and the number of nerve cells. Pearson Correlation test results can be seen in Table 4.
Discussion
The Increase of MLKL Expression after Administration of LPS This study showed an increase in MLKL expression after administration of P. gingivalis LPS for 24 hours. This study is in line with the previous study, which showed that LPS induction could activate the necroptosis cell death pathway by determining the proteins, including RIPK3 and MLKL, which increased after 24 hours of LPS administration [11]. The increase of MLKL expression in the P. gingivalis LPS treatment group occurred due to the activation of the Myeloid Differentiation Factor 88 (MyD88) pathway. This pathway is activated due to the presence of LPS detected by TLR-4 receptors on nerve cells in the dental pulp tissue. Through the MyD88 pathway, it triggers the activation of IL-1R-associated kinase (IRAK) signal transduction, which recruits TNFR-associated factor 6 (TRAF6). Furthermore, there is an activation of Nuclear Factor kappa of activated B cell (NF-κB), which will increase TNF-α. TNF-α leaves the cell and re-enters the cell, and binds to the TNF receptor (TNFR). This necroptosis pathway is in line with previous research where P. gingivalis LPS was administered to male rats in a periodontitis experiment and found increased expression of MLKL, which has specific properties in necroptosis [12]. In another study using P. gingivalis LPS, the LPS signal will be received by TLR-4 receptors on oral epithelial cells and regulate necroptosis by identifying at the expression of proteins associated with necroptosis, such as RIPK3 and MLKL, which have increased [13]. This is also in line with other studies, where a signal received by the TLR-4 receptor will induce the MyD88 pathway and necroptosis [14].
The Decrease of Number of Dental Pulp Nerve Cells after Administration of LPS
This study demonstrated that when P. gingivalis LPS was administered, there was a reduction in the quantity of nerve cells in the pulp tissue. The injection of P. gingivalis LPS resulted in necroptosis, which caused a reduction in the number of nerve cells. Necroptosis in nerve cells is caused by the activation of the Myeloid Differentiation Factor 88 (MyD88) pathway, which occurs due to the P. gingivalis LPS detection by the TLR-4 receptor, which plays a role in the activation of the MyD88 pathway. This study is in line with previous studies in which necroptosis can occur by the reception of the LPS signal by TLR4 and the involvement of the TLR/MyD88 pathway in nerve cell necroptosis, which increases during the inflammatory response in neurodegeneration [15,16]. MLKL oligomerization is then supported by the creation of necrosome complex during the necroptosis process, which happens via this route. Necroptosis takes place when the MLKL oligomer form translocates to the plasma membrane and creates a pore. Ion inflow, cell enlargement, and membrane lysis occur, and internal materials including damage-associated molecular patterns (DAMPs) are then uncontrollably released into the surrounding tissue. Necroptotic cells can attract inflammatory cells to assess the extent of tissue damage and eliminate any remaining necroptotic cell remnants when DAMPs are present in the tissue environment. This reduces the number of nerve cells in the tooth pulp tissue. Necroptosis causes a reduction in the number of nerve cells, which is consistent with the other research showing that the release of intracellular components can cause neuroinflammation and neurodegeneration [17]. Necroptosis is triggered by cell autonomic mechanisms that occur in nerve axons, and this is needed for the degeneration of astrocyte cells in nerves when inflammation occurs in degenerative neurological diseases, such as Amyotrophic Lateral Sclerosis (ALS) [18].
The Correlation between MLKL and Number of Dental Nerve Cells
The findings of this investigation showed that, although reducing the total number of nerve cells, nerve cell necroptosis in dental pulp tissue raised MLKL expression. Based on the negative correlation between the two, it can be said that when necroptosis happens, MLKL rises and the number of nerve cells decreases. This is consistent with other research that found that elevated MLKL expression and necroptosis-induced cell death caused nerve cells in nerve axons to deteriorate [18]. According to earlier research on Parkinson's disease, the phosphorylated MLKL would then translocate to the plasma membrane, cause membrane rupture and necroptosis, which is connected to neuronal death brought on by neurotoxins [19]. Furthermore, in another study, necroptosis occurs when an injury to the sciatic nerve occurs. MLKL, known as pseudokinase increases, can break the cell membrane during necroptotic cell death by targeting the Schwann cell membrane to induce damage to the myelin sheath [20-25]. Porphyromonas gingivals lipopolysaccharide administration increased the MLKL expression and decreased the number of nerve cells in necroptosis of dental pulp tissue after 24 hours.
Conclusion
To sum up, this study elucidates the impact of Porphyromonas gingivalis lipopolysaccharide (LPS) administration on dental pulp tissue, specifically focusing on necroptosis based on the expression of Mixed Lineage Kinase Domain-Like Protein (MLKL) and the number of nerve cells. The findings reveal a significant increase in MLKL expression coupled with a notable decrease in the quantity of nerve cells following LPS injection. This substantiates the induction of necroptosis in dental pulp tissue, pointing to a correlation between LPS exposure, heightened MLKL expression, and the ensuing reduction in nerve cell count. The observed necroptotic response underscores the potential implications for pulp quality deterioration and a consequential decrease in the likelihood of endodontic flare-ups. This research enhances our understanding of the mechanisms underlying LPS-induced necroptosis in dental pulp nerve cells, contributing valuable insights for future considerations in endodontic therapies.
Acknowledgements
There was no particular grant from a public, commercial, or nonprofit organization for the current research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Galih Sampoerno conducted research and is the owner of the frame work; Widya Saraswati and Hasna Shabrina carried out manuscript editing; Nathania Nurani Fripertiwi conducted research and Sarah Aniqoh Fauzya carried out statistical analysis.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Orcid:
Galih Sampoerno: https://orcid.org/0000-0003-1437-3185
Widya Saraswati: https://orcid.org/0000-0002-3459-8288
Hasna Shabrina: https://orcid.org/0000-0003-4252-3806
-------------------------------------------------------------------------------------
How to cite this article: Galih Sampoerno*, Widya Saraswati, Hasna Shabrina, Nathania Nurani Fripertiwi, Sarah Aniqoh Fauzya, Necroptosis after lipopolysaccharide administration based on expression of mixed lineage kinase domain-like protein and number of nerve cells in dental pulp tissue. Medicinal and Pharmaceutical Chemistry Research, 2024, 6(2), 236-244. Link: http://jmpcr.samipubco.com/article_184113.html
-------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
