Document Type : Original Research Article
Authors
- Galih Sampoerno 1
- Agustina Restu Nurkhotimah 2
- Arvia Diva Firstiana 2
- Naufal Hafidz Adipradana 3
- Adipradana Adipradana 3
1 Department of Conservative Dentistry, Faculty of Dentistry Airlangga University, Surabaya, Indonesia
2 Conservative Dentistry Specialist Program, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia
3 Undergraduate Program, Faculty of Dentistry Airlangga University, Surabaya, Indonesia
Abstract
The identification of dental pulp tissue may impact the risk of post-endodontic discomfort, including flare-ups. The amount of pulp tissue in teeth may influence the diagnosis of such tissue. Live pulp tissue reacts to pain more strongly than inflamed pulp. Three groups of 27 Sprague Dawley rats were used in the experimental study: a control group, a group that removed normal pulp tissue, and a group that removed inflammatory pulp tissue. Samples were taken from the mandibular incisor's apical field after the pulp tissue was extracted. Immunohistochemical techniques were employed for the examination. Before pulp tissue is extracted, LPS is administered, which causes the pulp tissue to become inflamed and results in a rise in Heat Shock Protein 70-expressing cells. Because of its overexpression, )Heat Shock Protein 70 (HSP-70( inhibits TRAF-6. Through the MAPK pathway, the drop in TRAF-6 led to a decrease in Nav1.8. When inflammatory pulp tissue is extracted, there is an overexpression of HSP70, leading to the inhibition of TRAF6. Consequently, this inhibition results in a decrease in MAPK, which subsequently lowers Nav1.8.
Graphical Abstract
Keywords
Introduction
Dental health issues still demand urgent attention because they have an impact on a person's quality of life. However, many people continue to give dental and oral health issues little thought. The World Health Organization (WHO) estimates that 560 million children have caries in their primary teeth and that over 2.3 billion adults have caries in their permanent teeth (WHO, 2017). More than 2 billion individuals throughout the world suffer from dental and oral health issues, and caries in primary teeth ranks 10 th among the most prevalent conditions that frequently occur [1].
Irreversible pulpitis is among the inflammations that may develop when dental caries in the pulp tissue are left untreated [2]. Root canal therapy, which includes consecutive steps including root canal preparation, sterilization, and obturation, is the main treatment for irreversible pulpitis [3]. The term "flare-up" during root canal procedures describes the severe pain and/or abscess that may develop and pose serious issues that necessitate an emergency dental appointment [4]. Extreme discomfort and edema following endodontic therapy that call for an urgent consultation and immediate active treatment are referred to as flare-ups. Injury types like mechanical and chemical are frequently linked to iatrogenic variables that might lead to flare-ups [5]. Morse et al. found that after treating asymptomatic pulp necrosis and chronic apical periodontitis, there was a 20% chance of flare-ups, which were characterized by swelling [6]. Nair et al. mentioned that women are more prone than men to have flare-ups, with a 2% frequency in individuals between the ages of 40 and 60. Only 1.9% of patients receiving a single-visit root canal experience flare-ups, which is extremely rare [7].
Anaerobic bacteria such Prevotella intermedia, Treponema denticola, Porphyromonas gingivalis, Fusobacterium nucleatum, and other gram-negative bacteria are mostly responsible for the inflammatory processes in pulp. Porphyromonas gingivalis is present in 48% of teeth that have tissue infections in the pulp and periapical areas [8]. Lipopolysaccharide (LPS) is attached to the wall of the Porphyromonas gingivalis bacteria. The hydrophobic domain (endotoxin) of lipid A, the hydrophilic O antigen, and polysaccharides make up the outermost layer of the membrane of gram-negative bacteria that transports LPS [9]. Toll-like receptors 4 (TLR4) that amplify LPS will set off a chain reaction that will ultimately cause inflammation [10]. When LPS attaches to TLR4, Myeloid Differentiation Primary Response Gene 88 (Myd88) is triggered. Next, Myd88 initiates the transduction of IL-1R-associated kinases (IRAK) signal, which activates tumour necrosis factor (TNF)-receptor-associated factor 6 (TRAF6) [11]. TRAF6 is a ubiquitin E3 ligase that acts as a mediator between various receptor types for endogenous or exogenous drugs and the activation of transcriptional responses that follow through the NFKB and MAPK pathways. The members of the MAPK kinase family are ERK, p38, and JNK. TRAF6 plays a role in JNK and p38 activation via several MAP3Ks. The JNK and p38 MAPK pathways' MAP3K is called ASK1. Furthermore, the two other MAP3Ks, TAK1 and MAPK-ERK kinase (MEKK)1/3, are activated in part by TRAF6 [12].
In excitable cells, voltage-gated sodium (Nav) channels are necessary for impulse generation and transfer. Nine distinct Nav channel subunit types are found in mammals. For example, subunits Nav1.3, 1.7, 1.8, and 1.9 are linked to neuropathic pain, whereas subunits Nav1.7, 1.8, and 1.9 are linked to inflammatory pain. Numerous voltage- and ligand-gated ion channels expressed in the main dental afferent neurons are potential sources of dental pain. Nav1.8 is present only in primary afferent neurons. Since Nav1.8 has been linked to neuropathic pain, pulpitis pathogenesis may be influenced by its expression in the tooth pulp. The main reasons for afferent nerve sensitization and the start of pain are thought to be changed expression and redistribution of Nav1.8, following nerve injury or inflammation [13]. Because of these channels' distinct distribution inside nociceptive neurons, pain treatment techniques are drawn to them. The Nav1.8 sodium channel is implicated in both pathological and normal nociception, as evidenced by genetic deletion and antisense therapy targeting this channel. The pathophysiology of dental pain may be impacted by Nav1.8 because it is expressed in the pulp of teeth. Neuropathic pain is linked to Nav1.8 [14].
The inflammatory response that LPS triggers is mediated by mitogen activated protein kinase (MAPK) signaling pathways [15]. Further research on a molecular basis is required to understand the flare-up because there are numerous reasons that can trigger flare-ups and the contradictory research results mentioned above. Under mechanical and bacterial stress to rat dental pulp tissue, neuroimmunological studies on flare-ups via the nuclear factor-κB (NF-κB) pathway have been conducted on nerve cells and macrophage cells [11]. This research uses the Immuno Histochemistry (IHC) method to examine the frequency of flare-ups in the dental pulp tissue (vital teeth) following extraction and in the dental pulp tissue treated with LPS (inflamed vital teeth). An immunological method was used in this work based on the expression of Nav18 and MAPK in the nerve cells.
Materials and methods
Samples
In this study, analysis of variance (ANOVA) was used to statistically examine the data from this post-test-only control group laboratory experiment. The Universitas Airlangga Faculty of Dental Medicine Health Research Ethical Clearance Commission examined and authorized all submitted procedures (certificate no. 621/HRECC.FODM/VIII/2022). A total of 27 Sprague Dawley rats were investigated, all of which had a clinical evaluation and were housed in a suitable environment for seven days. Aside from being in good health, the rats had to weigh 450g, be 20 weeks old, and have completely erupted incisors to be included in the study. NIH #31 rodent diet was used to provide the rats basic food.
Methods
In this study, three groups of 27 Sprague Dawley rats, each consisting of nine rats, were formed. The labels for these three groups were as follows: control group; group treated with extracted pulp tissue; and group administered LPS injection after pulp tissue extraction. In this investigation, three groups of 27 Sprague Dawley rats, each consisting of nine rats, were formed. Following were the labels assigned to these three groups: control group, the group that received LPS injection and pulp tissue extraction, and the group that received pulp tissue extraction.
Under no medical supervision, the rats in the control group were also put down at the same time. The rats were injected intraperitoneally with ketamine (80 mg/kg) and xylaxine (10 mg/kg) in sterile PBS to produce anesthesia in their mandibular incisors after their pulp tissue had been extracted. The rats were placed on a jaw retraction board and their teeth were cut at the level of the interdental papillae (3 mm) using a high-speed handpiece equipped with a fissure bur (Dia-Burs TC-21 ISO 160/014 FG LOT D14G007800 MANI Inc 8-3 Kiyohara Industrial Park Utsunomia, Tochigi, Japan). Next, the pulp chamber was opened using a high-speed handpiece (Panamax NSK OM-T0307E Japan) and a round bur. The rat's teeth were extracted using a barbed broach (VDW Gmbh-Bayerwaldstr. 1581737 Munich, Germany), which was rotated 360 degrees after it entered the root canal to remove the pulp tissue. The cavity was subsequently filled with glass ionomer cement and sealed after a day to prevent oral bacteria contamination.10 μl of Porphyromonas gingivalis-isolated LPS stock (Ultrapure lipopolysaccharide from Porphyromonas gingivalis - TLR4 ligand, Catalog # tlrl-ppglps. Version #14F18-MM. In vivo Gen. 3950 Sorrento Valley Blvd.) was given to the group administered LPS after pulp tissue extraction. The sample (Suite 100 San Diego, CA 92121 - USA) was put into the dental pulp chamber using a micropipette. The tooth was extracted following the 48-hour interval required for the induction of acute inflammation due to LPS, following the sealing of the cavity with glass ionomer cement [11]. After that, glass ionomer cement was used to cement the rat's teeth, which came out a day later.
Following the completion of pulp tissue extraction in every group, each jaw segment was declassified at 4% Diamine Tetraacetic Acid (EDTA) for 30 days in order to get it ready for the creation of paraffin blocks. It was fixed in 10% buffered neutral formalin for the following day. Then, tissue slices were roasted throughout the whole night at 56-58 °C in 4 μ thick paraffin blocks that were mounted on polysine slides. The incision was soaked in 3% hydrogen peroxide for 30 minutes at room temperature to eliminate endogenous peroxide activity from it. The 4-μ periapical tissue slices were deparaffinized in xylol and then rehydrated in a graded alcohol and water solution (20 minutes in xylol, 5 minutes in 100% alcohol, 5 minutes in 95% alcohol, and 5 minutes in 70% alcohol). Next, the tissue slices were washed under running water for five minutes. Following that, anti-rat monoclonal antibodies targeting Nav18 and MAPK were used for immunohistochemical staining. A 1000x magnification light microscope was used to evaluate the preparations, and digital camera images were captured [16].
Results
This section presents the findings from the investigation of the expression of Nav1.8 and MAPK following the extraction of tooth pulp tissue. The LPS + pulp tissue extirpation treatment group had the lowest mean MAPK expression, whereas the pulp tissue extirpation group had the greatest mean MAPK expression. Table 2 presents all treatment groups with p-value > 0.05 on the Shapiro-Wilk test. This means that all treatment groups have normal data distribution. The results of the Levene test showed that p > 0.05 means that the treatment group has homogeneity of variance (Tables 1-3).
The expression of MAPK differed significantly between the pulp tissue extraction treatment group and the control group, as demonstrated by the p < 0.05. The LPS + pulp tissue and pulp tissue extirpation groups and the LPS + pulp tissue extirpation and control groups were also shown to differ significantly from one another (Figures 1 and 2).
Table 4 indicates that there were nine samples in each group. The group that received LPS + pulp tissue extirpation had the lowest mean Nav1.8 expression, whereas the group that received pulp tissue extirpation had the greatest Nav1.8 expression.
As presented in Table 5, all treatment groups have a value of p > 0.05 on the Shapiro-Wilk test. This means that all treatment groups have normal data distribution. Levene test results showed that p > 0.05 means that the treatment group has a homogeneous variance.
The expression of Nav1.8 differed significantly between the pulp tissue extraction treatment group and the control group, as demonstrated by the p < 0.05. In contrast, there were significant differences between the groups that received LPS + Pulp tissue and pulp tissue extirpation.
There were nine samples total for each group, as indicated in Table 4. The LPS + Pulp tissue extirpation group had the lowest mean Nav1.8 expression, whereas the pulp tissue extirpation group had the greatest Nav1.8 expression.
Histological observation results
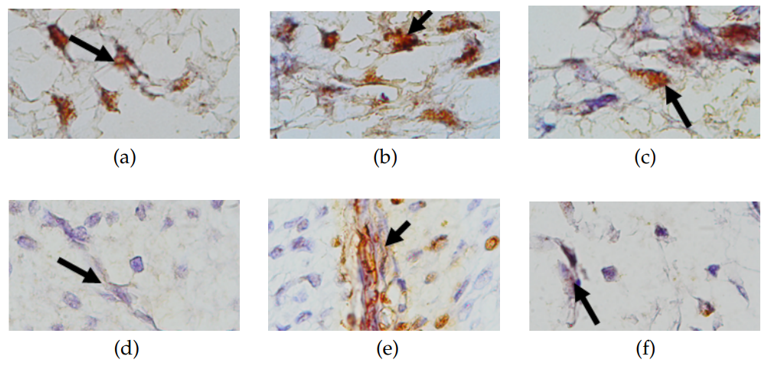
To observe the neuronal (nerve) cells of the pulp, Hematoxylin-Eosin staining was carried out so that it appears that the neuron cells in the cell nucleus will be blue while the cytoplasm and processes are red (pink). On immunohistochemical examination, neurons expressing positive MAPK and Nav1.8 were indicated by the presence of brown color in the cytoplasm and processes (photographed on an Olympus CX31 light microscope). An overview of MAPK and Nav1.8 expression is displayed in Figure 3.
(1000x magnification), (b) MAPK expression of normal pulp tissue extirpation treatment group (1000x magnification). (c) MAPK expression of inflamed pulp tissue extirpation treatment group (1000x magnification), (d) Nav1.8 expression of control group (1000x magnification), (e) Nav1.8 expression of normal pulp tissue extirpation treatment group (1000x magnification), and (f) Nav1.8 expression of inflamed pulp tissue extirpation treatment group (1000x magnification).
Discussion
MAPK and Nav1.8 intensify after pulp tissue extirpation in normal pulps
In this study, the dental pulp tissue removal was linked to an upregulation of MAPK and Nav1.8 expression which is due to the emergence of extracellular HSP-70, which is generated immediately after cell death, i.e. during the extrusion of pulp tissue. After recognizing HSP-70 as an endogenous molecule that might develop as a result of tissue damage, TLR4 interprets it as a danger-associated molecular pattern (DAMP), which triggers TLR4 activation. The adaptor protein is then used by TLR4 to recruit MyD88 to the cytoplasmic TIR domain. Serine/threonine kinases, namely TRAK4, IRAK1, IRAK2, and IRAK, as well as the development of myddosome, a multiprotein complex made up of MyD88. The intricate configuration starts the signaling pathway that is dependent on MyD88. TRAF6 is then recruited by IRAK activation, which is the consequence of intracellular signal transduction. A mechanism that occurs downstream of TRAF-6 is TRAF6 via MAPK pathway. The phosphorylation cascade of MAP3K, MAPKK, and MAPK regulates the MAPK pathway, activating MK and the transcription factor AP-1. The MAP3K activation is mediated by TRAF6 and is primarily driven by JNK MAPK and p38. By taking away thioredoxin, TRAF6 triggers ASK1 MAP3K in an oxidative stress scenario. The p38 MAPK activation involves ASK1 and TAK1. There is a rise in Nav1.8 expressing cells as a result of MAPK activation, which also causes the expression of Nav1.8 sodium channels. This is in line with the findings of the study in [11], which showed that after pulp was removed, macrophages increased in TNFa and HSP70 in response to physical damage during extraction. TLR4 on the surface of macrophage cells recognizes the damage. This sets off the major differentiation response Myd88, which attracts TRAF6 by activating IRAK through intracellular signal transduction. Moreover, Cuadrado and Nebreda's study, which found that TRAF6 ubiquitin ligases play a significant role in TAK1 activation. TAK1 activation often mediates p38 MAPK activation triggered by cytokine receptors [17]. This has been connected to the Lys63-linked polyubiquitin chain's function as a scaffold for the complex assembly necessary for kinase activation. More recently, TRAF6 is necessary for the activation of p38 MAPKs (and JNKs) by TGF β receptors [18]. TGF β-induced auto-ubiquitination of TRAF6 is facilitated by TRAF6's contact with the TGF β receptor, and this connection is necessary for TRAF6's affiliation with and subsequent activation of TAK1 [19]. TRAF6 controls TAK1 activation without relying on the TGF β receptor's kinase activity or the traditional Smad pathway. Importantly, in response to TNF α stimulation, TAK1 also functions as a key activator of the NF-kB anti-apoptotic pathway in addition to MAPK. Members of the TNF receptor family, including CD40, must activate MAP3Ks like MEKK1 or TAK1 by forming a multiprotein complex on the intracellular domain of the receptor. Moreover, sodium channels Nav1.8 are expressed as a result of MAPK activation, which raises the number of cells expressing NaV-1.8. Numerous VGSC isoforms with varying sites and functions have been found in the tooth pulp. In the inflamed pulp, only Nav1.7, Nav1.8, and Nav-1.9 were significant players. According to the study by Zheng et al., this sodium channel isoform was suggested as a possible target as a novel therapeutic for inflamed pulp pain and as a novel anesthetic alternative in the pulpitis treatment. Since it has been established that Channel Nav1.8 is important for human pain signaling, this study demonstrates that the presence of damage from pulpal extirpation treatment can exacerbate the host's discomfort.
MAPK and Nav1.8 reduction after pulp tissue extirpation in inflamed pulps
The LPS administration caused a decrease in MAPK and Nav1.8, and the over-expression of HSP-70 caused the pulp tissue to be extracted. This is due to the fact that gram-negative bacteria produce LPS, a chemical that damages pulp tissue. TLR4 identifies these pathogen-associated molecular patterns (PAMPs). In the initial phase of infection, it activates Myd 88, and then triggers signal transduction. intracellular resulting in activation of IRAK. Activated TRAF-6 triggers the underlying inflammatory pathway, this condition causes the release of HSP-70. HSP-70 is also expressed due to pulp extirpation treatment plus when there is cell death there is separation of HSP 70 and HSF due to the presence of a signaling pathway under activated TLR-4. HSP 70 as Capherone goes to the target cell while HSF moves from the cytosol to the cell nucleus to join with other HSF monomers to form HSF trimer. The HSF trimer is then related to the connector in the heat shock protein (DNA) gene, this bond produces HSP messenger RNA (mRNA), the mRNA will move to the cytosol where a translation process occurs which ultimately forms HSP 70 protein. There is an accumulation of increased HSP-70 for the reasons stated above. Increased expression of HSP locally can cause an increase in the concentration of this protein in serum. Extracellular HSP-70 interacts with membrane receptors and activates inflammatory pathways. HSP-70 exhibits anti-inflammatory effects on the intracellular environment and pro-inflammatory effects on the extracellular environment. This explains how the pulp condition that experiences over-expression of HSP-70 due to LPS treatment and extirpation actually reduces the degree of inflammation because HSP-70 has an anti-inflammatory effect. The HSP-70 overexpression inhibits inflammatory activity. By interacting with TRAF-6, intracellular HSP-70's anti-inflammatory characteristics can prevent LPS-mediated NFkB activation. There will be a rise in cells expressing HSP-70 if the introduction of LPS creates inflammation in the pulp tissue prior to the tissue extraction. TRAF-6 is inhibited by HSP-70 due to its overexpression. Through the MAPK pathway, the drop in TRAF-6 led to a decrease in Nav1.8.
Conclusion
To sum up, the findings of this molecular analysis shed light on the intricate interplay between Nav1.8, MAPK, HSP70, and TRAF6 in dental pulp tissue under various conditions. The observed increase in Nav1.8 and MAPK expressions in healthy dental pulp tissue following the removal of pulp tissue underscores the dynamic nature of these molecular pathways in maintaining homeostasis. In contrast, in inflamed dental pulp tissue, the HSP70 overexpression emerges as a key regulatory mechanism, acting through the inhibition of TRAF6. This inhibition cascades down to reduce MAPK, subsequently resulting in the Nav1.8 downregulation. The significant implication of these findings lies in their potential clinical relevance, particularly in the context of root canal therapy. The data suggest that the removal of infected pulp during root canal procedures may not only address the source of infection, but also contribute to a reduction in pain perception. By understanding the molecular mechanisms involved in the modulation of Nav1.8 and MAPK, clinicians may be better equipped to manage pain associated with dental pulp inflammation. However, it is important to acknowledge the complexity of pain perception and the multifaceted nature of the molecular pathways involved. Further research is warranted to explore the broader implications of these findings and to elucidate additional factors that may influence pain outcomes in dental pulp tissues. Nonetheless, this study provides valuable insights into the molecular dynamics of dental pulp and offers a foundation for future investigations aimed at refining therapeutic strategies for managing dental pain effectively.
Acknowledgements
There was no particular grant from a public, commercial, or nonprofit organization for the current research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Galih Sampoerno: conduct research and owner of the frame work; Agustina Restu Nurkhotimah: manuscript editing; Arvia Diva Firstiana: manuscript editing; Naufal Hafidz Adipradana: statistical analysis.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Orcid:
Galih Sampoerno: https://www.orcid.org/0000-0003-1437-3185
Agustina Restu Nurkhotimah: https://www.orcid.org/0000-0002-7901-4039
Arvia Diva Firstiana: https://www.orcid.org/0000-0003-4225-7907
-----------------------------------------------------------------------------------------
How to cite this article: Galih Sampoerno*, Agustina Restu Nurkhotimah, Arvia Diva Firstiana, Naufal Hafizh, Adipradana, Expression of mapk and nav-1.8 in nerve cells in normal and inflamed pulp after dental pulp tissue extirpation. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(3), 245-254. Link: http://jmpcr.samipubco.com/article_184185.html
-----------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
