Document Type : Original Research Article
Authors
- Ria Ramadhani Dwi Atmaja 1
- Fidia Rizkiah Inayatilah 1
- Mawar Yuli Muktisari 1
- Safa'atun Ni'mah 1
- Imam Subandi 2
1 Department of Pharmacy, Faculty of Medicine and Health Science, Universitas Islam Negeri Maulana Malik Ibrahim, 65144, Malang Indonesia
2 Biomedical Laboratory, Departement of Medicine Universitas Islam Negeri Maulana Malik Ibrahim, 65144, Malang, Indonesia
Abstract
The plant known as Red fruit oil (Pandanus conoideus Lamk.) has the ability to accelerate wound healing. Red fruit oil contains compounds that are active in the healing process of incisional wounds, including tocopherols, carotenoids, oleic acid, linoleic acid, and linolenic acid. To enhance stability and penetration, red fruit oil was formulated in an emulsion form in this research. The aim of this study was to determine the activity of red fruit oil emulgel on collagen density and the amount of angiogenesis in incisional wound healing and to determine the optimal dose of red fruit oil emulgel on collagen density and the amount of angiogenesis in incisional wound healing. This research is a true experimental study that employs a post-test-only control group design with 6 treatment groups. ImageJ software is used to measure the percentage of collagen density area, while image raster software is used to measure angiogenesis. This study has shown that red fruit oil emulgel can enhance collagen production and angiogenesis in incisional wound healing. The optimal dose concentration of red fruit oil emulgel to increase collagen density and the amount of angiogenesis in incisional wound healing was shown in red fruit oil emulgel with a concentration of 15% w/v. The conclusion is that red fruit oil emulgel leads to an increase in collagen density and angiogenesis during wound healing in rat incisions.
Graphical Abstract
Keywords
Main Subjects
Introduction
The definition of a wound is when body tissue is damaged due to injury. Wounds are classified into three types: the cause, the presence or absence of tissue loss, and the degree of contamination [1]. An incised wound caused by a sharp object is one of the most common types of wounds [2]. Incisional wounds or injuries frequently occur in everyday life, particularly in the industrial and household sectors, as indicated by Riskesdas data from 2018. Based on the national injury prevalence in Indonesia, incisional wounds have a percentage of 20.1%, where this data ranks third (after abrasions and sprains) as the most common cause of national injury prevalence. Several endogenous factors in the human body, such as age, immunity level, metabolic conditions, nutritional intensity, and medication use, impact the wound-healing process. In addition, there are numerous histopathological factors that assist in the process and mechanism of wound healing. Histopathological factors that are present include fibroblasts, collagen amount, and epithelial thickness, white blood cell count, angiogenesis, and other variables. The wound-healing process is influenced by collagen, which is one of the most important factors [3]. Collagen production is triggered by increasing levels of Transforming Growth Factor-ß (TGF-ß), which can increase the fibroblast proliferation mechanism, thereby increasing the number of fibroblasts. These fibroblasts have a function in forming collagen and extracellular matrix so that they increase the synthesis of extracellular matrix collagen which accelerates the synthesis of connective tissue and microscopic blood vessels on the wound surface. Therefore, the faster collagen synthesis occurs, the faster the wound will close and heal [4]. In addition to collagen, the process of creating new blood vessels is also significant and commonly known as angiogenesis. The mechanism of angiogenesis is the formation of new blood vessels from pre-existing blood vessels by surrounding the blood clot and forming into the microvasculature along the granulation tissue where this occurs in the proliferation phase [3].
Povidone iodine is currently being used by the public as their first line of treatment for wound healing. However, it is known by research that the use of povidone-iodine as a wound treatment can cause excessive irritation and form scar tissue and there is potential for severe irritant contact dermatitis and chemical burns associated with the use of povidone-iodine as an antiseptic [5]. To overcome this condition, one of the choices is to use herbal medicines considered to be capable of treating wounds efficiently, such as applying red fruit oil [6]. Red fruit oil is still used empirically to treat incisional wounds. Red fruit oil is well-known for its ability to reduce inflammation and boost immune cell production. Increased immunity, the inactivation of free radicals in the skin, and the synthesis of new collagen fibers are all critical for speeding up the healing process and restoring the integrity of the skin tissue after an injury. These benefits can be obtained from the active ingredients in red fruit oil [7].
Since applying red fruit oil topically to treat incisional wounds is thought to be less effective, more workable formulations are required, one of which might be an emulgel formulation [8]. When the emulsion and gel are combined in an emulgel formulation will be a preferred option in drug delivery systems since it has an advantage over other formulations in terms of spreadibility, adhesion, viscosity, and extrusion. Along with all the benefits listed above, they will also work as a solution to load hydrophobic medications into water-soluble gel bases, giving them a suitable medium [9]. Thus, the purpose of this study was to ascertain how well red fruit (Pandanus conoideus Lamk.) oil gel emulsion affected the amount of angiogenesis and collagen density in incisional wound healing in rats (Rattus norvegicus).
Experimental
The research design that is being employed is a True Experimental study conducted in a laboratory setting using the Post Test Only Control Group Design research method. This means that measurements or post-tests are conducted after the variables have undergone an intervention. This method is used to determine the relationship between cause and effect by administering therapy to experimental animals as study subjects and then examining the effects of the treatment.
Plant and chemical materials
The materials used in this research were red fruit oil from CV Made Mulya, Papua Indonesia, normal saline 0.9%, liquid ketamine and xylazine, povidone-iodine 10%, xylol, 96% alcohol, distilled water, hematoxylin paint, HCl, and eosin paint.
Tools
The tools used in this study were sawdust husks, plastic food containers, drinking water bottles and gram scales, microscope, Image J 1.52V application, image raster application version 3.0, SPSS application version 17.0, digital camera, terumo 1 injection syringe ml, ruler, dry patch, sterile gauze, plaster, hair clipper, scalpel (scapel), razor (gilet), surgical scissors, tweezers, pins, glass preparation, gloves, and mask.
Preparation of red fruit oil (MBM) emulgel
Red fruit oil emulgel formulations are divided into three formulations containing different active ingredient concentrations: 5% w/v (F1), 10% w/v (F2), and 15% w/v (F3). This MBM formula also uses excipients such us carbomer as a base of emulgel, propylene glycol as an enhancer, ethylparaben, and propylparaben as preservatives, and butyl hydroxytoluene (BHT) as an antioxidant. The result of physical characterization of MBM emulgel shown in Table 1.
Preparation of animal-tested
In this study, randomization or grouping of experimental rat (Rattus norvegicus) was divided into six treatment groups using male balb/c strain rat. The six groups are positive control group (K+), negative control (K-), group P1, group P2, group P3, and group P4. Each group consisted of 5 rat. Rat were anesthetized first using a combination of ketamine and xylazine intramuscularly at a dose of 1: 1. Next, the hair on the back was shaved using a razor, and then the area where the wound would be made was marked using a marker. The next step, an incision wound 4 cm long with a depth of 0.2 cm is made using a scalpel knife. All procedures carried out in this study involving experimental animals have been ethically appropriate in accordance with 7 the World Health Organization (WHO) standards with certificate number of passing the ethical test:
Reg.No.:738 / KEPK-POLKESMA/ 2022. After the incision was made, red fruit oil emulsion was given to each treatment group with the following details. K- was given gel emulsion base (carbomer), K+ was given Povidone iodine 10% v/v, P1 was given red fruit oil, P2 was given emulsion red fruit oil gel 5% w/v, P3 was given red fruit oil gel emulsion 10% w/v, P4 was given red fruit oil gel emulsion 15% w/v. The emulgel preparation was given to the wound on the rat's back in the amount of 0.5 grams so that it could cover the wound surface completely and absorb into the center of the wound. This is done for 14 days with a frequency of administration 2 times a day, given in the morning and evening.
Histopathological microscopic observations
Collagen density was observed using an Olympus microscope equipped with an optilab with 400x magnification in five fields of view [10]. Observations were made on the six treatment groups observed histopathologically with the ImageJ 1.52V application with the final data results in the form of area percentages. Calculation of the amount of angiogenesis was carried out using 5 fields of view on an object with a magnification of 400 times. The calculation results are averaged and the results are compared with other treatments.
Data analysis
The analysis used SPSS 25 for Windows where the Shapiro-Wilk normality test and Levene's Test of homogeneity were carried out. Next, the data was tested using the One Way Anova test and the Post Hoc Fisher Least Significant Difference (LSD) test.
Results and Discussion
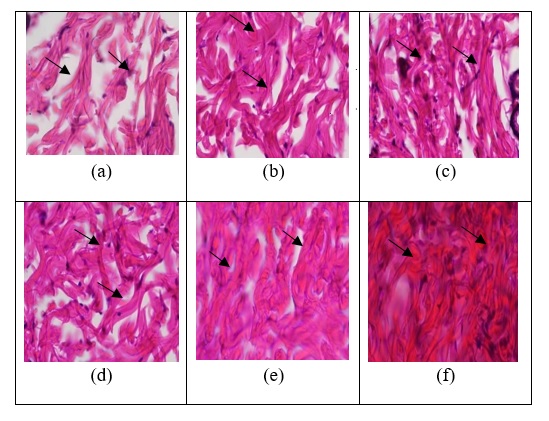
Inflammation, proliferation, and maturation are the three stages of wound healing [11]. Two things were discovered in this study, collagen density and the amount of angiogenesis. The first effect is increased collagen density, which can be seen histologically in Figure 1. Based on Figure 1, the distribution of collagen density in each treatment group shows that the negative control group (K-) had the lowest collagen density among the other groups, with numerous fibroblasts marked with a purplish blue color and few collagen fibers produced [12]. The negative control group (K-) is still in the initial proliferation phase, while the positive control group (K+) and treatment groups (P1, P2, P3, and P4) experienced more accelerated healing, as proven by the large number of collagen fibers in the group that has reached the final proliferation phase. The low collagen density in the negative control (K-) group was also evident in the mean percent area results, with the negative control group (K-) having the lowest mean percent area among the other groups. Wound healing can occur naturally. When an injury occurs, the cell membrane of the skin is broken, which accelerates arachidonic acid metabolism. The cyclooxygenase pathway's digestion of arachidonic acid culminates in the release of inflammatory mediators, notably prostaglandins. Prostaglandin release promotes an inflammatory reaction in the injured skin [4]. As a result, reducing pain in inflammatory wounds is essential for facilitating healing and patient comfort. This pain can be reduced by the activity of active chemicals that operate to speed the passage of the inflammatory phase, consequently accelerating the proliferative phase in which the collagenization process occurs, the production of fibrin threads, and the formation of wound-covering tissues.
However, because the negative group (K-) lacks active chemicals, the process of generating new tissue, including collagenization, takes longer and yields a lower proportion of collagen area. Meanwhile, the positive control group (K+), which received 10% povidone-iodine ointment, had more collagen fibers and a greater average percent area than the negative control group (K-). However, no significant differences were found between the negative control group (K-) and the positive group (K+) based on average collagen density and Post Hoc LSD results. This is due to the knowledge that 10% povidone-iodine functions as an antiseptic that can kill gram-positive and gram-negative bacteria, including those that are resistant to antibiotics, fungi, yeast, viruses, and protozoa. As a result, using 10% povidone-iodine in wound treatment can increase the density of the collagen in the wound because the inflammatory process ends quickly, but the collagenization process takes a long time to increase because there is not an active ingredient that can cause the collagenization process in large quantities [13].
When compared to the positive group, there was a bigger difference in the percentage of collagen density in the treatment group provided pure red fruit oil (P1) and red fruit oil emulsion in treatments 2 (P2), 3 (P3), and 4 (P4). (K+). This is because the antibacterial activity of 10% povidone-iodine does not stimulate collagen synthesis in large quantities. Additionally, povidone-iodine has a negative effect in that it can inhibit the growth of fibroblasts, thereby reducing collagen synthesis [10]. The utilization of red fruit oil as the active ingredient in the healing of incisional wounds has been found to be efficacious in expediting the inflammatory and proliferation phases of wound healing. This is attributed to the presence of fatty acids, specifically oleic acid, linoleic acid, and linolenic acid. Oleic acid can accelerate the inflammatory phase by removing infections, and preventing further tissue damage [14]. Oleic acid can also induce Transforming Growth Factor-beta 3 (TGF-3) to create type 3 collagen is vital at every stage of wound healing because it can replace damaged or destroyed tissue [15]. Linoleic acid is an omega-6 fatty acid that acts as a precursor to arachidonic acid, producing prostaglandins, thromboxane, and leukotriene. This chemical works as an inflammatory mediator, which might hasten the inflammatory process [16]. Prostaglandins not only have the power to increase inflammation, but they also have the therapeutic potential to rebuild macrophage tissue [17]. The thromboxane produced induces vasoconstriction and platelet aggregation, resulting in blood clotting. Meanwhile, leukotrienes attract neutrophils to the wound site via infiltration in order for them to perform phagocytosis of incoming foreign substances [16].
Based on the Post Hoc LSD test results, it is possible to conclude that there is a significant difference (p-value 0.05) between treatment groups. The average findings of group P1 (pure red fruit oil) and the emulgel treatment groups (P2, P3, and P4) differ significantly. This suggests that administration of red fruit oil emulgel at 5%, 10%, and 15% concentrations have activity in enhancing collagen density for faster healing of incisional wounds. Treatment group 1 (P1) has the lowest average percentage of collagen density compared to the other treatment groups because high oil content inhibits the diffusion of active chemicals into the skin. Meanwhile, because the emulgel preparation could permeate the skin efficiently, treatment groups P2, P3, and P4 showed better collagen density outcomes. This is due to the presence of excipients or other substances in the MBM emulgel formulation. Propylene glycol serves as an enhancer in the formulation of this MBM emulgel product. An enhancer is a component that has the capacity to increase or decrease skin permeability. Although they do not have a therapeutic impact, penetration-enhancing compounds can help medications travel more easily through the skin, facilitating the correct delivery of the active ingredient to the wound center [5]. The saturated fatty acid concentration in red fruit oil easily reacts with air, causing an increase in the development of off-flavor (rancidity) and the loss of bioactive components [18]. This can reduce the activity of the active component in red fruit oil, resulting in poorer collagen density results when compared to the treatment group that has been formed into emulgel (P2, P3, and P4). Excipient substances such as methylparaben and propylparaben as preservatives, as well as BHT as an antioxidant agent, are added to the MBM emulgel preparation to boost the stability of the MBM emulgel preparation so that active substance content is not harmed and can perform effectively.
Quantitative analysis of collagen density is indicated in Figure 2. with the average area percentage results.
According to the Post Hoc LSD test results in Figure 2, there is an average difference in the area percent results between MBM emulgel formulations at P2, P3, and P4, where the p-value between P2 and P4 is significantly different, but less significantly different from P3. According to the findings, raising collagen density by administering red fruit oil emulgel at a concentration of 15% has a faster collagenization activity than giving red fruit oil emulgel at concentrations of 5% and 10%. According to the certificate of analysis (CoA) of red fruit oil, every 1 ml includes 120 ppm carotenoids, 100 ppm tocopherol, 35.81 ppm beta carotene, 14.6 ppm β-cryptoxanthin, 13.68 ppm α-tocopherol, 7.46 ppm% oleic acid, 0.08% linoleic acid, and 0.83% linolenic acid. This indicates that the red fruit oil emulgel, which in this trial included 15% w/v of dosages, contains more active ingredients to stimulate the process of collagen production. Dosing accuracy in the therapeutic when providing medication will offer optimal therapeutic results [19]. Besides, treatment with the emulgel dosage form can affect the wound healing process, where the emulgel dosage form can increase stability and make it a dual control release system so that the release of active substances in the emulgel is better than the topical drug delivery system [9].
The next observation result is the average amount of angiogenesis. Five fields of view of an object magnified 400x were used to calculate the amount of angiogenesis. The computed outcomes are averaged and contrasted with those of other therapies. Figure 3 displays the observation findings, while Figure 4 depicted the average results.
Based on the result, the quantity of angiogenesis was lowest in the negative control group (K-), which was simply administered gel base. This is due to the gel base's inability to directly stimulate the creation of new blood vessels, as it simply serves as a transporter and delivery material for active compounds into the wound. Meanwhile, during wound healing, angiogenesis is formed through a series of intricate interactions between the cells involved, such as endothelial cells and inflammatory cells that require active components [20].
When compared to the treatment group, the positive control group (K+) that received 10% povidone-iodine therapy in the form of an ointment showed much less angiogenesis. This is because Povidone iodine 10% can decrease fibroblast development while also stimulating angiogenesis growth factors. Povidone iodine 10% has been shown to decrease the expression of Vascular Endothelial Growth Factor (VEGF), which is essential for the growth and proliferation of blood vessel cells, and limit endothelial cell proliferation in vitro cell cultures [21]. Povidone-iodine 10% additionally delays epithelial cell migration on the wound surface and increases the number of inflammatory cells that collect in the wound, interfering with scar tissue development [22]. The absorption of the active component in skin incision wounds in rats was hampered because the ointment's sticky and oily character could interfere with the wound-healing process by causing skin irritation and making it difficult to apply to the skin hairy places, preventing the active ingredient from being efficiently absorbed by the skin [23].
The positive control (K+) and negative control (K-) groups had lower average levels of angiogenesis than the P1 group, but the P1 group's average was not higher than that of the P2, P3, or P4 groups. Red fruit oil's big molecular size makes it difficult for it to permeate the skin's dermis and epidermis layers. Its high viscosity also makes it challenging for the oil to spread evenly throughout the skin and absorb into pores [24]. This causes the active components in red fruit oil to be poorly absorbed in the wound, causing the active substances that should aid in the production of angiogenesis to be inhibited and underperform. To address red fruit oil's molecular and viscosity issues, researchers are developing emulgel formulations that can aid in reducing oil molecule size and enhancing the penetration of active chemicals into the skin [25].
Red fruit oil is a red fruit extract that includes unsaturated fatty acids, particularly linoleic and oleic acids, β-tocopherol, and beta-carotene as active ingredients [7]. Based on the Gas Chromatography (GC) study, one of the most active ingredients in red fruit oil (79.7%) is unsaturated fatty acids. Unsaturated fatty acids can promote angiogenesis. Specifically, endothelial cells' receptors are activated by omega-3 and omega-6 unsaturated fatty acids, which in turn stimulate the production of angiogenesis factors and influence the neovascularization process. When this receptor is activated, signaling pathways that result in the production of angiogenesis factors including VEGF and FGF are also activated [26].
Red fruit oil contains linoleic acid, which has an effect on incision wounds. According to the study, linolenic acid aids in the creation of blood vessel growth factor VEGF and angiogenic factor proliferative, moving endothelial cells. Increased angiogenesis and cell citation are often the results of higher amounts of linoleic acid interacting with increased growth factors like VEGF [26]. Increased VEGF and angiopoietin 2 (ANGPT-2) expression during the proliferative phase can lead to an increase in the number of blood vessels in wound tissue [27]. Oleic acid is another fat component that is an active chemical. The activation of fatty acid receptors on the surface of endothelial cells by oleic acid can lead to an increase in Akt1 and Akt2 activity, thereby initiating an intracellular signaling cascade [28]. After that, vascular growth factor (VEGF) is expressed more when protein kinase serine/threonine 1 and 2 (Akt1 and Akt2) is activated, which increases angiogenesis [28]. Apart from the high fatty acid content, red fruit oil contains another active substance, namely the active substance α-tocopherol. The control of chemical signals in endothelial cells, or the cells that make up blood vessel walls, is one method. α-tocopherol can alter the expression of genes involved in the angiogenesis process, such as VEGF. These two growth factors are crucial in the establishment of angiogenesis. Thrombin-1 and endostatin are two examples of angiogenesis inhibitory factors whose production can be inhibited by α-tocopherol. Both proteins are known to impede the angiogenesis process. Red fruit oil includes both β-carotene and α-tocopherol, which is the active ingredient. One carotenoid with an angiogenic impact and aids in the healing of incisional wounds is β-carotene [29].
Conclusion
Red fruit oil emulgel (Pandanus conoideus Lamk.) exhibits an optimal dose concentration of 15% and can increase angiogenesis and collagen density in wound healing in rats (Rattus norvegicus). Although red fruit is designed in emulgel formulation with a better penetration level than emulsion and gel formulation, it is assumed that the presence of carotenoids, tocopherols, oleic acid, linolenic acid, and linoleic acid plays a significant role in this effect on wound healing.
Acknowledgments
The authors would like to thank to Drs. I Made Budi, M.Si from Cenderawasih University, Papua, for his assistance in providing Red Fruit Oil.
Orcid:
Ria Ramadhani Dwi Atmaja: https://www.orcid.org/0009-0004-5803-0744
Fidia Rizkiah Inayatilah*: https://www.orcid.org/0000-0001-7157-3760
Mawar Yuli Muktisari : https://www.orcid.org/0009-0002-9329-1929
Safa'atun Ni'mah: https://www.orcid.org/0009-0003-9426-5607
Imam Subandi : https://www.orcid.org/0000-0003-0115-0740
-------------------------------------------------------------------------------------
How to cite this article: Ria Ramadhani Dwi Atmaja , Fidia Rizkiah Inayatilah* , Mawar Yuli Muktisari , Safa'atun Ni'mah , Imam Subandi , The potential of red fruit oil emulgel (pandanus conoideus lamk.) in accelerate the proliferation phase of rat incision wound healing. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(3), 302-313. Link: http://jmpcr.samipubco.com/article_184703.html
-------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
