Document Type : Original Research Article
Authors
1 Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2 Departement of Child Health, Dr. Soetomo Public Academic Hospital, Surabaya, Indonesia
3 Departement of Clinical Pathology, Dr. Soetomo Public Academic Hospital, Surabaya, Indonesia
Abstract
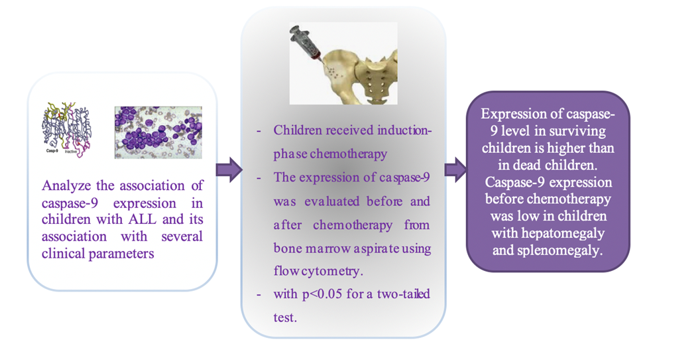
Caspase-9 plays a critical role in mediating apoptosis through various intrinsic pathway triggers within the mitochondria. Failure to respond to apoptotic stimuli in Acute Lymphoblastic Leukemia (ALL) can lead to chemotherapy resistance related to deviations in the caspase pathway in lymphoblasts. This study aims to assess the caspase-9 expression both before and after the induction phase of chemotherapy in children with ALL and its association with several clinical parameters. This prospective study involved pediatric patients aged 1-18 years who were newly diagnosed with ALL and administered induction-phase chemotherapy following the 2018 Indonesian National Protocol for ALL treatment. The expression of caspase-9 was evaluated before and after the induction phase from bone marrow aspirate using flow cytometry. The variance in caspase-9 expression was examined using the Mann-Whitney U test, with p < 0.05 for a two-tailed test was considered statistically significant. Out of the 37 pediatric ALL cases, 17 met the specified criteria. The median expression of caspase-9 before chemotherapy in dead children was higher (p = 0.182). The median expression before chemotherapy increased after the induction phase of chemotherapy (p = 0.561). The caspase-9 expression was low in children with hepatomegaly and splenomegaly (p = 0.015, p = 0.009). Expression of caspase-9 was elevated after chemotherapy and higher in surviving children than in dead children. Caspase-9 expression before chemotherapy was low in children with hepatomegaly and splenomegaly.
Graphical Abstract
Keywords
Introduction
Acute lymphoblastic leukemia represents the most prevalent form of cancer in children. While survival rates range from 70% to 90%, the challenges of chemotherapy resistance and relapse continue to persist [1,2]. The mortality before chemotherapy occurred in 22% and 10.5% in the first two weeks of chemotherapy. Acute lymphoblastic leukemia accounted for 91.2% of children with hematology malignancy and 44.4% of overall cancer children in East Java from 2014 to 2015 [3]. The 5-year free survival rate of only 36% from 223 children with ALL from 2006 to 2011. Meanwhile, the outcome of post-induction remission was only 46.4% of 143 children diagnosed with ALL between 2013-2014 using the 2013 Indonesian ALL protocol. Chemotherapy failure was associated with failure to induce lymphoblast apoptosis, especially in children with initial leukocytes of more than 50,000/µL, high-risk ALL, and T-cell leukemia [1,4]. A combination of chemotherapy protocol and new drugs for cancer developed over time, but failure to achieve remission and relapse remain challenges [1,5-7].
An imbalance in the expression of proapoptotic and antiapoptotic proteins has been linked to the outcomes of chemotherapy, distinguishing between remission and non-remission in pediatric ALL [8,9]. Apoptosis can occur through either the extrinsic pathway, which is receptor-mediated, or the intrinsic pathway, which is mediated by mitochondria [10]. Caspases, which belong to a family of cysteine proteases with specificity for aspartate, play a central role as both initiators and executioners of apoptosis. Apoptotic caspases are classified into two categories: initiator or apical caspases (caspase-2, caspase-8, caspase-9, and caspase-10) and effector or executor caspases (caspase-3, caspase-6, and caspase-7) [11].
Intrinsic pathway apoptosis is mediated by caspase-9 via mitochondria [4,12-13]. Caspase-9 is essential in various intrinsic pathway stimuli in mitochondria, including its role in chemotherapy. Failure to activate caspase-9 will impact the formation of degenerative processes and the development of cancer [14].
Decreased caspase-9 expression has been reported in reducing apoptosis, which is associated with poor outcomes and chemotherapy resistance in multiple cancers [15,16].
However, caspase-9 expression in lymphoblast in children with ALL has not been investigated to date. This study aims to assess the caspase-9 expression before and after the induction phase of chemotherapy in children with ALL and to determine whether the caspase-9 expression is correlated with several clinical parameters that are evaluated, for example, age, gender, laboratory results, and outcomes.
Methods
This prospective study was carried out on children aged 1-18 years who were newly diagnosed with ALL. The research was conducted at the Hematology Oncology Ward, Department of Child Health, Dr. Soetomo Public Academic Hospital, Surabaya, East Java, Indonesia, spanning from October 2020 to March 2021. Ethical approval for the study was obtained from the Health Research Ethics Committee, and a letter of exemption was granted under Ref. No. 0116/LOE/301.4.2/IX/2020. Demographic data collected were age and gender. Reported signs and symptoms of leukemia are fever, pallor, bleeding, bone pain, hepatomegaly, splenomegaly, and lymphadenopathy. Complete blood count evaluation was evaluated using the Sysmex® XP-100 Hematology Analyzer. Hemoglobin level is grouped into hemoglobin less than 8.0 g/dL, 8.0-10.0 g/dL, and more than 10.0 g/dL. The platelet count is grouped into platelets less than 50x109/L, 50-150x109/L, and above 150x109/L. The number of leukocytes is grouped into leukocytes less than 4x109/L, 4-20x109/L, 21-50x109/L, and more than 50x109/L. The neutrophil count is categorized as neutropenia when it falls below 0.5 x 109/L.
The diagnostic criteria for ALL involve identifying the presence of lymphoblasts in bone marrow aspiration (BMA) smears, which should account for 20% of the 200 nucleated cells examined. A pediatric hematology oncology consultant read the BMA results. Pediatric ALL is classified into subtypes known as ALL-L1, ALL-L2, and ALL-L3 based on lymphoblast morphology, following the French-American-British (FAB) Classification. Children with ALL-L3 or those under 12 months of age were excluded from this study, as they received chemotherapy with a different treatment regimen. Immunophenotyping was employed to assess the molecular characteristics of lymphoblasts using flow cytometry, which was conducted on 0.5-1 mL bone marrow aspirate samples via FACS Calibur. The markers used for this evaluation included CD34 markers for early hematopoietic (both myeloid and lymphoid) cells, B lymphocyte markers (CD10 and CD20), T lymphocyte markers (CD3, CD5, and CD7), and myeloid markers (CD13, CD33, MPO, and HLA DR). Examination of caspase-9 expression is only carried out on samples with confirmed B-cell and T-cell leukemia [17].
Pediatric ALL was managed by the 2018 Indonesian ALL Chemotherapy Protocol, which was formulated by the Working Group of Hematology-Oncology of the Indonesian Pediatric Society. The treatment approach encompassed two distinct chemotherapy regimens, dependent on risk classifications: standard-risk ALL (SR-ALL) and high-risk ALL (HR-ALL). Patients aged above ten years, with a leukocyte count at diagnosis exceeding 50 x 10^9/L, and those with mediastinal tumors or central nervous system metastases were categorized as high-risk cases. During the induction phase of chemotherapy for standard-risk ALL (SR-ALL), the regimen consisted of the following components: (1) Vincristine at a dose of 1.5 mg/m2 administered intravenously on days 8, 15, 22, 29, 36, and 43, (2) Methotrexate given intrathecally at a dose of 12 mg on days 8, 22, and 36, (3) Daunorubicin administered intravenously at a dose of 25 mg/m2 on days 22 and 29, (4) L-asparaginase at a dose of 7500 units/m2 given intravenously on days 36, 38, 40, 42, 44, and 46, and (5) Prednisone at a dose of 60 mg/m2 for seven days, followed by 40 mg/m2 orally in three divided doses from day 8 to day 42, with a gradual tapering over seven days. For high-risk ALL (HR-ALL), prednisone was replaced with dexamethasone at a dose of 6 mg/m2. Additionally, the dose of daunorubicin was increased to 30 mg/m2 on days 8, 15, 22, and 29, and L-asparaginase was administered at a dose of 7,500 units/m2 intravenously on days 29, 32, 35, 38, 41, 45, 48, and 51. After the seventh week, bone marrow aspiration was performed to evaluate the chemotherapy response [18]. Patients who do not continue chemotherapy will be excluded from this study. The outcomes obtained were death, remission, and no remission. Remission was defined as the presence of fewer than 5% lymphoblasts in the bone marrow aspirate out of the 100 nucleated cells examined. Caspase-9 expression within lymphoblasts was assessed using the flow cytometry method, employing bone marrow aspirate samples collected in EDTA tubes. The analysis was carried out with a FACS Calibur instrument from Becton Dickinson, and data were processed using the Cell Quest software package (Becton Dickinson). The required bone marrow aspirate sample volume ranged from 0.5 to 1 mL and was collected both before and after the induction phase of chemotherapy. Intracellular staining for caspase-9 expression was conducted following the manufacturer's instructions. The reagent utilized for Anti-caspase-9 Antibody (96.1.23) Alexa Fluor 647, namely sc-56076, was sourced from Santa Cruz Biotechnology, Inc. in Oregon, USA. The evaluation of caspase-9 expression in lymphoblasts occurred at least 6 hours after the sample collection and was conducted in the Clinical Pathology laboratory at Dr. Soetomo Public Academic Hospital. Caspase-9 expression was quantified as a percentage. The primary data was entered into the Statistical Package for the Social Sciences (SPSS 19.0) software for subsequent analysis. Paired data of caspase-9 expression were evaluated using the Wilcoxon Signed Rank Test. Differences in protein expression between survival groups were assessed through the Mann-Whitney U Test. For comparisons of proportions between two unpaired groups, the Fisher exact test and Kolmogorov-Smirnov tests were applied. In all tests, a confidence level of p < 0.05 was used, and a two-tailed test was employed.
Results
Since October 2020 to March 2021, a total of 37 children with suspected leukemia were examined. Twenty children were excluded from the study, including 4 who did not exhibit lymphoblasts, 7 with myeloid lineage immunophenotyping, 6 who lacked baseline data, 2 with incomplete data, and one who declined chemotherapy. The number of children with ALL who met the criteria was 17 subjects. In this study, we compared 11 (65%) LLA children who lived until the end of observation and 6 (35%) children who died as research subjects. All of the children who died had symptoms of pale and fever (p = 0.515 and p = 1,000). The proportion of deaths in children who had bleeding was greater and statistically significant (70.6% compared to 29.4%, p = 0.043). No significant differences in mortality were observed based on hemoglobin levels, leukocyte count, platelet count, and absolute neutrophil count, as summarized in Table 1. Out of the total number of children, 16 out of 17 were diagnosed with ALL-L1, while only one was diagnosed with ALL-L2. The specific data can be found in Table 2. Among the children, four deaths occurred in those with ALL-L1. In B-cell leukemia cases, there were 5 out of 6 deaths (83.3%), whereas in T-cell leukemia, there was 1 out of 6 deaths (16.6%). However, the difference in the proportion of deaths did not reach statistical significance. The death rate was higher in SR-ALL compared to HR-ALL, with a p-value of 0.304. The causes of death among the six children included infection (3 cases), hemorrhage (1 case), pneumonia (1 case), and tumor lysis syndrome (1 case).
The median expression of caspase-9 at the beginning of diagnosis in 11 children's ALL was 29.7% lower than after chemotherapy was 32.8% (p = 0.561). The median expression of caspase-9 at the beginning of diagnosis or before chemotherapy in the 11 ALL children who lived was 29.7%, and in the six children who died was 10.5% with p = 0.182, as presented in Table 3. Variations in clinical parameters of pediatric ALL before the induction phase of chemotherapy are detailed in Table 4. Expression of caspase-9 in children aged more than ten years was higher than those aged 1-10 years (p = 0.159). Boys expressed lower levels of caspase-9 than girls, but it was not significantly different (p = 0.159). Children with hepatomegaly and splenomegaly expressed lower levels of caspase-9. The mean expression of caspase-9 in children with and without hepatomegaly was 21.9% compared to 62.8% (p = 0.015). The expression of Caspase-9 was also lower in children with splenomegaly, namely 11.4% compared to 44.6% (p = 0.009). Caspase-9 expression did not differ significantly based on haemoglobin classification and platelet count (p = 0.634 and p = 0.052). Caspase-9 expression did not differ significantly depending on the leukocyte count and absolute neutrophil count (p = 1.000 and p = 0.304). Caspase-9 expression before the induction phase of chemotherapy was higher in ALL-L1 compared to ALL-L2, although this difference was not found to be statistically significant (p = 0.329). Caspase-9 expression was lower in B-cell leukemia compared to T-cell leukemia and SR-ALL compared to HR-ALL, but both were not statistically significant (p = 0.090 and p = 0.634), as listed in Table 5.
Discussion
In the last decade, advances in the management of pediatric ALL have led to notable improvements in chemotherapy outcomes, although the mortality rate remains relatively high. We found that caspase-9 expression was lower in children with leukemia who died. The expression of Caspase-9 was also down in children with hepatomegaly, splenomegaly, and low platelets. The proportion of ALL boys in this study was more remarkable than that of girls, as is the case in other literature [19-23].
Children aged 1-10 reached 82.4%, with a median age of 6.75 years. The number of leukemia cases in children aged < 1 year is 5.1% and 62.3% aged 1-10 years [19]. Activation of caspase-9 will encourage granulocytic differentiation of leukemia cells. Failure of caspase-9 activation is also associated with uncontrolled leukocyte growth [23].
The most common complaints are fever, pallor, bleeding, bone pain, hepatomegaly, splenomegaly, and lymphadenopathy [25]. The most common laboratory abnormalities in this study were anemia (41.2%), thrombocytopenia (70.6%), and leukopenia (45.5%). The initial laboratory examination supposes the leukemia diagnosis if we found leukocytosis with lymphocyte predominance plus bi-cytopenia (anemia and thrombocytopenia) [5,22]. Case of ALL-L1 predominates compared to ALL-L2 based on FAB criteria (93.8%). Other studies report varying results [5]. Most of them were categorized as B cell ALL as per reports from the literature and also in adult ALL [26-28].
The number of children with high risk and standard risk was almost the same, according to the Indonesian Pediatrician Society (Indonesia ALL Chemotherapy Protocol 2018). The risk classification for ALL also refers to the Children's Oncology Group (COG) based on age, leukocyte count, cytogenetics, bone marrow response on day 14, and late induction of minimal residual disease [29]. The death rate during the induction phase of chemotherapy was 35.3%, but all of the children achieved complete remission. Research in Bangladesh on pediatric ALL showed a greater mortality rate of 15.16% due to febrile neutropenia and failure to achieve remission of 14.13% after induction phase chemotherapy [30].
Chemotherapeutic agents work primarily by inducing apoptosis. At the cellular level, apoptosis is regulated by the receptor-mediated extrinsic and mitochondria-mediated intrinsic pathways [1]. Caspase-9 expression in children's ALL before induction phase chemotherapy was 29.7%. Caspase-9 was lower in patients with lung cancer and acute myeloid leukemia [31]. Caspase-9 expression before chemotherapy increased after chemotherapy, but did not differ significantly based on lymphoblast morphology, immunophenotyping, and risk classification. Ex vivo evaluation of lymphoblast cultures reported that apoptosis occurred in 3-29% before chemotherapy, but apoptosis increased significantly by 1-38% or 1.5-4.7 times from the start of chemotherapy [14]. This study only evaluated caspase-9 as the initiator or promotor of caspase of the intrinsic apoptotic pathway. The study did not evaluate the final or upstream results of the caspase cascade (caspase-3) and did not evaluate caspase-8 as an initiator caspase in the extrinsic pathway. The median expression of caspase-9 in this study at the beginning of diagnosis in children with survival outcomes was much higher than in children who eventually died, namely 29.7% compared to 10.5%. Failure to activate caspase-9 will impact the formation of degenerative processes and the development of cancer [14]. Caspase-9 activity is low in lymphoblasts before receiving resveratrol chemotherapy in vitro but increases 4-6 times after mitochondrial changes occur 48 after resveratrol administration [32]. The ratio of increase in caspase-9 after chemotherapy is 1.6 times with an increased range of 0.33-6.35 times. The increase in caspase-9 expression after chemotherapy indicates the role of apoptosis in the success of chemotherapy. Chemotherapy in pediatric ALL is a combination and works on various apoptotic pathways. Chemotherapy drugs induce intrinsic apoptosis, activating caspase-9 in the apoptosome [33]. Caspase-9 expression before induction phase chemotherapy showed no significant difference when classified based on the initial leukocyte count. One of the used drugs is dexamethasone. Caspase-9 expression was in the range of 7.8% to 97.1%. Dexamethasone will increase caspase activity in cell cultures, especially caspase-8, caspase-9, and caspase-3. Caspase-3 expression also increased in pediatric acute lymphoblastic leukemia after the induction phase of chemotherapy [34]. Dexamethasone causes direct damage to mitochondria in lymphoid cells, resulting in caspase-mediated apoptosis, especially caspase-9 [35]. This study could not evaluate the apoptosis rate, either spontaneous or chemotherapy-induced. Caspase-9 plays a role in increasing the susceptibility to cancer but also sometimes plays a role in reducing the risk of cancer [35].
Increasing the concentration of anti-cancer peptide will increase the expression of caspase-9 by 1.7 times and caspase-3 by 1.8 times at the same peptide dose [13]. Expression of caspase-9 before chemotherapy between B cell ALL and T cell ALL showed no difference. The expression of caspase-9 in T cell ALL appears to be higher than in B cell ALL before chemotherapy. Caspase activity increased 4 hours after exposure to daunorubicin, a chemotherapy agent in treating pediatric ALL. The increase occurred in cultured T-cell leukemia and B-cell leukemia cells, but the increase in caspase activity in T-cell leukemia was higher than in B-cell leukemia [10]. Caspase activity will increase after chemotherapy. Doxorubicin and daunorubicin have also been shown to stimulate increases in caspases-8 and caspase-9 [10,37]. Caspase-9 activation failure and an elevated apoptotic threshold were observed in the resistant cells [38].
The difference in expression of caspase-9 in children who lived and those who died was three times greater, but statistically, the difference was not significant (p = 0.182). It indicates that the children who died had problems initiating apoptosis. Failure of apoptosis initiator (caspase-9) will cause loss of activation of caspase executor (caspase-3). However, the absence of caspase-9 initiates a compensatory pathway that includes caspase-8 [11-13,39]. Therefore, future research should evaluate other caspases, especially caspase-3 and caspase-8. The relationship between the intrinsic pathway via caspase-9 and the extrinsic pathway via caspase-3 and caspase-8 was not studied. Children with death outcomes had low expression of caspase-9 before chemotherapy. Failure of apoptosis may occur due to caspase-9 gene polymorphism. Caspase-9 polymorphisms are associated with poor outcomes in leukemia [15]. Caspases-9 can be used as prognostic biomarkers for tumor susceptibility. Caspase-9 polymorphisms are often related to the function of the produced protein, and their expression is often absent. Polymorphisms may not be detected in the outcomes or analyses [11].
To the best of our knowledge, this is the first study that has aimed at differences in the expression of caspase-9 levels in lymphoblast before and after chemotherapy in children with ALL. Our results found that expression of caspase-9 increased after chemotherapy, indicating apoptosis probably due to chemotherapy. Furthermore, expression of caspase-9 in children alive was also higher than in children who died, showing that apoptosis occurs more in children alive. All participants who completed the induction phase of chemotherapy achieved remission during this phase. However, this study did not evaluate apoptosis itself. The apoptosis process can occur spontaneously or chemotherapy-induced. Further research is needed to validate these findings and to examine the other proteins related to caspase-9 and the apoptotic index.
Conclusion
Caspase-9 protein expression is not related to the results of induction phase chemotherapy (remission and non-remission) in pediatric ALL because all children who completed chemotherapy experienced remission. Caspase-9 expression before chemotherapy was higher, especially in children with hepatomegaly and splenomegaly. Caspase-9 expression in lymphoblasts before chemotherapy showed no difference between ALL children who were still alive and those who had died, even though the expression in children who were alive was three times greater. In children who were still alive and had undergone chemotherapy, the expression of caspase-9 before and after the induction phase also showed no difference.
Acknowledgments
The authors extend their gratitude to all the staff in the Division of Hematology-Oncology and the Pediatrics Residents at the Faculty of Medicine, Universitas Airlangga/Dr. Soetomo Public Academic Hospital, Surabaya, East Java, Indonesia
Disclosure Statement
The authors have affirmed that there are no conflicts of interest about the research and the composition of this publication. The main researcher will utilize this publication to fulfill the graduation requisites for the Specialist Medical Education Program as a pediatrician at the Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia.
Funding
This research was not financially supported by any sponsors.
Ethical Declaration
Ethical approval for this research was obtained from the Health Research Ethics Committee of the Clinical Research Unit (CRU) at Dr. Soetomo Public Academic Hospital, Surabaya, East Java, Indonesia, with the reference number 0116/LOE/301.4.2/IX/2020.
Authors' Contributions
Adecya Nararia Anindita, MD
She serves as a research resident in pediatrics at the Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia. Her contributions encompassed conceptualization, methodology, manuscript composition, data collection, and analysis.
E-mail: [email protected]
Telephone: +6282141122486
Orcid number: 0009-0001-8737-2880
I Dewa Gede Ugrasena, MD, Ph. D (Corresponding author)
He holds the position of Professor in the Hematology Oncology Division within the Department of Child Health at the Faculty of Medicine, Universitas Airlangga/Dr. Soetomo Public Academic Hospital, Surabaya, East Java, Indonesia. His role involved supervising and reviewing the manuscript.
E-mail: [email protected]
Telephone: +62818334593
Orcid number: 0000-0002-0458-7801
Yetti Hernaningsih, MD, Ph. D
She serves as a Clinical Pathologist and is also the Head of the Department of Clinical Pathology at the Faculty of Medicine, Universitas Airlangga/Dr. Soetomo Public Academic Hospital, Surabaya, East Java, Indonesia. Her contributions encompassed supervising data collection, caspase-9 examination, and reviewing the laboratory aspect.
E-mail: [email protected]
Telephone: +6281332269754
Orcid number: 0000-0001-8773-8267
Andi Cahyadi, MD
He serves as a pediatric hematology-oncologist within the Hematology Oncology Division at the Department of Child Health, Faculty of Medicine, Universitas Airlangga/Dr. Soetomo Public Academic Hospital, Surabaya, East Java, Indonesia. His contributions included conceptualization, methodology, manuscript composition, and data analysis.
E-mail: [email protected]
Telephone: +6281334633097
Orcid number: 0000-0002-4523-3663
Data availability statement
The data that underpins the findings of this study can be obtained from the corresponding author upon a reasonable request.
Orcid:
Adecya Nararia Anindita: https://www.orcid.org/0009-0001-8737-2880
IDewa Gede Ugrasena*: https://www.orcid.org/0000-0002-0458-7801
Yetti Hernaningsih: https://www.orcid.org/0000-0001-8773-8267
Andi Cahyadi: https://www.orcid.org/0000-0002-4523-3663
--------------------------------------------------------------------------------------
How to cite this article: Adecya Nararia Anindita, I Dewa Gede Ugrasena*, Yetti Hernaningsih, Andi Cahyadi, Expression of caspase-9 in bone marrow lymphoblasts in children with acute lymphoblastic leukemia. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(3), 314-326. Link: http://jmpcr.samipubco.com/article_184843.html
--------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
solid tumors in children in East Java, Asian J Heal Res, 2023, 2, 27−33. [Crossref], [Google Scholar], [Publisher]

.png)
.png)
.png)
.png)
