Document Type : Original Research Article
Author
Department of Mathematics and Statistics, College of Science, University of Jeddah, Jeddah, Saudi Arabia
Abstract
Medical researchers face a significant hurdle such as time commitment, large amount of costs, establishing a safety profile for medications, reduced solubility, and insufficient experimental data while producing innovative drugs. By studying the structural properties of molecules, chemical graph theory plays a significant role in advancing drug development and design. To improve drug research and assess the effectiveness of treatments, degree-based topological indices play a crucial role in Quantitative Structure-Property Relationship (QSPR) analysis, aiding in the estimation of their properties and potential efficacy. In this article, ten reducible degree-based topological indices are calculated and QSPR analysis for 15 stroke drugs (Atenolol, Baclofen, Dapsone, Diclofenac, Dopamine, etc.) to correlate with seven physicochemical properties (Molar mass, Melting point, XlogP, Complexity, LogP, Vapor pressure, and Solubility) using linear regression model. This methodology enhances drug discovery and development for strokes by offering valuable insights into the correlation between molecular structure and pharmacological properties. This theoretical technique might help chemists and pharmaceutical industry workers to predicted stroke drug properties without expensive testing.
Graphical Abstract
Keywords
Introduction
Before the discovery or manufacture of antibiotics, the lives of both humans and animals were seriously in danger of bacterial infection. Until Alexander Flemings' 1929 discovery of penicillin, the first antibiotic was presented to the world in 1940. Before that time, various bacterial diseases were the most frequent causes of death. Among other infectious diseases, bacterial infections are the most frequent stroke-causing factor [1]. Stroke is one of the leading causes of death and morbidity in the whole world. It may be ischemic or hemorrhagic, with the former being brought on by a nearby thrombus or a distant embolus [2]. A stroke, also known as a brain attack, occurs when an artery limits blood supply to a brain region or a brain blood vessel ruptures, damaging or killing brain cells. The stroke effects can include permanent brain injury, sustained disability, or even loss of life. The part of the body that controls a particular function will not work properly if a stroke occurs and the area cannot receive enough blood flow. More than 795,000 people in the United States have a stroke each year [3].
Stroke has two main causes: Blood clots or other substances blocking the blood arteries to the brain cause a (ischemic stroke). Plaque, which is fatty deposits that occur in blood vessels, can also blocks flow burst or leakage of a blood artery (hemorrhagic stroke). Diseases such as hypertension and aneurysms (arterial bulges that may develop and burst like balloons) are among the many possible causes of hemorrhagic strokes. "Mini-stroke" (or transient ischemic attack) is a term used to describe a kind of stroke. Strokes of caution are another name for transient ischemic attacks [4]. A TIA is a medical emergency on scale with a stroke. More over a third of TIA patients who do not get medical care will have a massive stroke within a year. Between 10 and 15 percent of persons will have a massive stroke within three months after having a TIA.
It is crucial to remember that different stroke symptoms might appear, and that each person may have a different set of symptoms based on the type and stage of their stroke. Stroke happens when our brain does not get enough blood flow due to a blocked blood vessel or bleeding in the brain. The signs can be pretty scary. One of the most common symptoms is sudden numbness or weakness in our face, arm, or leg - especially if it's just on one side of our body. Sometimes we might have trouble speaking or understanding what others are saying. We might feel dizzy, lose balance, and coordination, or even experience a severe headache that feels like someone smacked you with a sledgehammer. Figure 1 represents the sudden symptoms of stroke disease.
In the branch of mathematical chemistry called chemical graph theory, several graph theory methods are applied to the mathematical modeling of chemical phenomena. Molecular networks are used to represent molecules and molecular compounds in models, with edges standing in for chemical bonds and vertices representing individual atoms [5]. A new academic discipline, "cheminformatics," was created from the combining of chemical and mathematical sciences with computer and information sciences. Quantitative structure-activity relationships (QSARs) and quantitative structure-property relationships (QSPRs) are discussed, both of which are used to predict the biological activities and features of different chemical compounds [6]. Quantitative structure activity relationship models can be used to predict various attributes of different chemical compounds using topological descriptors, avoiding this inconvenience. Topological indices recently discovered significant use in a number of mathematical chemistry applications, including isomer discrimination, chirality, molecular complexity, drug design, database selection, and QSAR/QSPR/QSTR research [7]. A graph's topological index is a number that accurately characterizes the graph's atomic topology and remains unchanged upon automorphism of the graph. Wiener [8] came up with the concept of topological indices while investigating the boiling point of paraffin (an alkane). This topological metric became known as the path number. As study of chemical graph theory progressed, the path number became known as the Wiener index. In 1975, [9] Randic introduced the Randic index, which was later extended by Bollobas and Erdos (1998). Gutman and Trinajstic (1972) created the first and second Zagreb indices almost 40 years ago. Predicting drug bioactivity is possible with the use of the ABC index, the Wiener index, and the Randic index. In this study, we calculated degree-based TIs for medicines used for the treatment of stroke. Likewise, medications used for the treatment of stroke are organic compounds with properly calculated topological indices that are analyzed by QSPR analysis. When utilizing linear regression, the characteristic of stroke medications and the characteristic calculated by this method have a strong correlation. The qualities of drugs and TIs have been determined to be strongly correlated [10]. According to [1], the SC-index is critical for estimating the enthalpy of heart attack medications based on their boiling point (r = 0.925) [11]. For more information about to calculate the physical properties of different medicines by theoretical method see [12-15].
The following describes the manner in which the article is organized: Topological indices and stroke medications are discussed in Section 1. The methods used to calculate the results relating to topological indices are all described in Section 2. Section 3 includes tables with experimental values and topological indices for stoke medicines. All calculations related to statistics are presented in Section 4. In Section 5, we provide a graphical evaluation of all available correlation coefficients. Section 6 explains how to use the topological indices presented in the article, and Section 7 analyzes the results. The bibliography is included at the conclusion of the work.
Preliminaries
If there is a connection between any two nodes in a graph Ω with vertex set V() and edge set E(), then the graph is said to be connected. The length of the shortest path between two nodes “i” and “k” in graph is used to denote the distance between them as . The "degree" of a particular node “k” is defined as the count of nodes of adjacent to it and where n=. This node is indicated by the notation , or if misunderstanding is not possible, simply using . The ideas of degree and valence in chemistry are partially interconnected.
Reducible First and Second Zagreb index
The Zagreb topological index is a graph-theoretical descriptor used in quantitative structure-property relationship (QSPR) studies and mathematical chemistry. It was introduced in 2000 by Matthias Randić. The Zagreb topological index has been shown to be useful in predicting various molecular properties, such as boiling points, toxicity, and biological activities of chemical compounds. It reflects the topological complexity and symmetry of a molecule, and its calculation is relatively simple, making it a valuable tool in molecular modeling and drug design.
Reducible Reciprocal Randic index
In 1975, Milan Randic released a paper titled "On Characterization of molecular branching" in which he first described the topological index as a molecular descriptor [16].
The Randic topological index is a tool used in graph theory to quantify the complexity and connectedness of molecules. The index is calculated by adding the square roots of each bond's distance, where distance is calculated as the product of the vertices' connecting degrees. The Reciprocal Zagreb index is another graph-theoretical descriptor used in chemical graph theory and molecular modeling. It is a variation of the Zagreb topological index, and it was proposed to address certain limitations of the original index.
Reducible Reciprocal Sum Connectivity index
In chemical graph theory, the "Reciprocal Sum Connectivity Index" (RSC) is a topological index used in quantitative structure-property relationship (QSPR) investigations. In 1995, Ivanciuc and Balaban introduced it. The Reciprocal Sum Connectivity Index (RSCI) of a graph Ω is the sum of the reciprocals of the shortest distances between every pair of vertices. In chemical graph theory, the RSCI has been utilized to model diverse molecular properties and activities of chemical compounds, such as boiling points, toxicity, and biological activities.
Reducible First and Second Hyper Zagreb index
It was introduced by Zhou and Gutman in 2006. The total square roots of all vertices that are not neighboring in a molecule create the First Hyper Zagreb index. This index provides us with important information on the relationships between the various atoms in a molecule. The First and the Second Hyper Zagreb indices are higher-dimensional extensions of the Zagreb index and provide more details about the distribution of vertex degrees in the graph.
Reducible Sigma Index
The Sigma index is a graph-theoretical descriptor used to study the structural properties of molecules. It was introduced by Gutman and Trinajstić in 1972. The Sigma index has applications in quantitative structure-property relationship (QSPR) studies and mathematical chemistry. It is used to model various molecular properties and activities of chemical compounds and provides details about the topological complexity and symmetry of a molecule.
Reducible Forgotten index
In 2015, Furtula and Gutman presented the reduced forms of the Zagreb indices [17]. The forgotten index is determined by adding up the squares of the vertices degrees. Using graph theory and topology principles, the structural characteristics are represented numerically by the forgotten topological index. This value takes into account various factors, including connectivity, atomic distances, and bond angles. By employing graph theory and topology, the forgotten index provides a comprehensive understanding of a molecule’s structural properties. Through the forgotten index, scientists and researchers gain valuable insights into the complex interplay of atoms within a substance. It serves as a powerful tool for analyzing and predicting the molecule’s properties, enabling a deeper comprehension of its behavior and potential applications. Inspired by the applications of this index, we present our innovative addition known as reducible forgotten index, which is mathematically defined as follow:
Reducible First Gourava and Second Gourava index
The first Gourava index of a molecular graph was introduced by V.R. Kulli [18], drawing inspiration from the original Zagreb indices and their wide-ranging applications. Building upon the concept of the first Gourava index and the use of generalized Zagreb index, Kulli also introduced the novel notion of the second Gourava index. He computed this index for several standard classes of graphs and further applied the formula's definition to armchair polyhex and zigzag-edge polyhex nanotubes. Using the concept of this index, we create a new form which is characterized through the reducible idea. These indices are mathematically defined as follow:
Methods and Strategies
Simple graphs are utilized to model the effects of stroke medications. Vertex partitioning, edge partitioning, and computational techniques are used to compute the topological indices of the structure of the drug under consideration.
Particularly Atenolol to Allopurinol, properties and computations of the chemical compounds used to treat stroke in terms of reducible topological indices. The practical values of the properties are taken from ChemSpider. Software such as Chem-Sketch, ChemDraw includes (Struct/Name, ChemDraw/Excel, and ChemNMR), Microsoft Excel and Biovia Draw can all be utilized for modeling of chemical structures. Microsoft Excel is used to create line graphs.
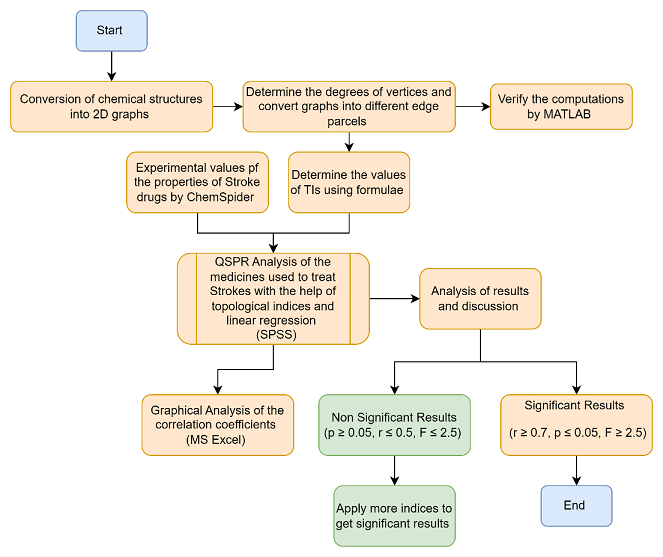
Analysis of the correlation coefficients can also be accomplished using the scatter plot. The results can be determined using Statistix, SageMath, and MATLAB. The best program for statistical calculations is SPSS. The whole procedure to estimate the properties of stroke drugs are given in Figure 2.
Topological Analysis of Stroke Drugs Structures
In this study, degree-based topological indices for the medications used for the treatment of stroke are generated. The QSPR analysis of the computed indices is described, and it shows that these indices have a strong relationship with the physicochemical characteristics of the medicines used for the treatment of stroke. The following drugs are included in a mathematical analysis for stroke disease: atenolol, baclofen, dapsone, diclofenac, dopamine, linezolid, phenytoin, thiotepa, melphalan, lorazepam, hydralazine, gabapentin, clobazam, amifostine, and allopurinol. Figure 3 demonstrates the molecular structure of various drugs. A graph is used to describe the chemical structure of a medicine, and the elements on the graph stand in for the vertices and bonds for the edges. Atenolol, a beta blocker, is utilized to treat arrhythmia and higher blood pressure (hypertension).
Atenolol assists individuals with elevated blood pressure in preventing future heart attacks, strokes, and other cardiovascular diseases. Baclofen is used to help relax certain muscles in human body. It eases muscle spasms, cramps, and tightness brought on by illnesses like multiple sclerosis or specific spinal injuries. When measured in people with or at risk of heart disease and stroke, allopurinol has been demonstrated to enhance peripheral vascular function. Gabapentin is used to treat stroke patients' nerve pain, which can be brought on by various illnesses, such as shingles and diabetes. For the treatment of high blood pressure, hydralazine can be taken with or without other drugs. Heart attacks, strokes, and kidney issues can all be avoided by lowering high blood pressure.
Edge division values are determined by topological indices to analyze structures. The edge degree and formulas are used to calculate all T-indices. After calculating the vertices' degrees, the edges' degrees are used to categories the nodes.
We have used here 10 degree-dependent T-indices, namely first Zagreb index M1(Ω), second Zagreb index M2(Ω), reciprocal randic index RR(Ω), reciprocal sum connectivity index RSC(Ω), first hyper Zagreb index HM1(Ω), second hyper Zagreb index HM2(Ω), sigma index S(Ω), forgotten index F(Ω), first gourava index G1(Ω), second gourava index G2(Ω) for modeling seven representative physical properties [Molar mass (MM), melting points(MP), XLOGP3, complexity, Log P, Vapour pressure (VP) and solubility(S)] of the 15 stroke drugs from atenolol to Allopurinol. Chem Spider utilized to obtain the values for these characteristics. The experimental data for the physical and chemical are presented in Table 1.
The edge and vertex numbers of molecular graphs of chemical structures are displayed in Figure 1. Let . We compute topological indices of chemical structures using the edge partition technique. Table 2 lists the topological index values for these structures.
Its edges can be used as divisions in the following ways:
Regression Models
One of the most popular modeling techniques is linear regression since it not only explains the connection between variables (like correlation), but also offers an equation that can be used to predict the value of a response variable based on the value of the predictor variable. For the medications, we complete a linear regression and a regression analysis. The following equation is used to link some mode to the various physical properties of various drugs used to treat strokes.
.png)
Where, p indicates for the physicochemical characteristic of the provided medication. Topological index, constant, and regression coefficient are all abbreviated as TI , A, and b, respectively. The discussed linear regression equation is used to define the regression model for the topological indices that had been taken into consideration. Physical characteristics of anti-stroke medications are considered as dependent variables, whereas topological indices for molecular graphs of 15 medications are considered as independent variables. To determine the constants a and b in the regression equation (1), a linear regression model is fitted using SPSS software and the training set from Tables 1 and 2 is used to derive these constants.
Determination of Statistical Parameters
This section investigates the physical properties of stroke drugs such atenolol and allopurinol in connection to degree-based TIs. Regression parameters have been computed to achieve this.
The sample size is N. A represents the constant or Y-intercept, b the slope, r the correlation coefficient, and the proportion of the dependent variable. A linear model's ability explains changes. While a lower (nonsignificant) p value tests the null hypothesis that the coefficient is equal to zero (no impact), a larger (insignificant) p value indicates that there is no association between changes in the predictor and changes in the responder.
Think about a test in which the assumption is that all of the regression coefficients are zero. The F value, which is a number, is the result of this kind of test. In this situation, the model is unable to make any predictions. By contrasting the model to one with no predictor variables, this test enables one to assess if their new coefficients have enhanced the model. In Tables 3-12, these statistical parameters for different T-indices have been computed for linear regression models.
Seven physical features were utilized to derive regression models and Tables 3-12 provide the correlation coefficients between these indices and the corresponding models (, , RSC, , , S, F, , and . Depending on the inverse nature and direct relationship between two variables, R values may be negative or positive. With correlation coefficients of 0.9465 and 0.9408, respectively, the QSAR analysis of the first Zagreb index demonstrates its use for the calculation of molar mass and complexity. The correlation for this measure varies from -0.3047 to 0.9465. Physicochemical characteristics of stroke medications are correlated with the index, as presented in Table 3: |r|= 0.3047, 0.9465, 0.1292, 0.5557, 0.9408, 0.4961, and 0.06337. It is defined that the index and molar mass have a significant correlation (r=0.9465). It is clear from Tables 4, 7, and 9 that , , and S() indices strongly correlate with the molar mass, with correlation coefficients of r = 0.9313, 0.9368, and 0.9449, respectively.
Tables 5, 6, 8, 10, 11, and 12 show that the complexity and the RR(), RSC(), HM2(), F(G), , and indices have a significant relationship, with correlation coefficients of 0.9435, 0.9511, 0.8849, 0.5304, 0.9351, and 0.9147, respectively.
In Table 10, the results of the Forgotten index's linear model are given. The correlation coefficients are between -0.158 and 0.5304. The range of p-values is 0.9018 to 0.04193. With the exception of complexity, every parameter has non-significant p values. Table 12 also shows that the G2() index has good correlations with molar mass, solubility, XlogP3, logP, melting point, and vapor pressure, with r= 0.8301 for molar mass, |r| = 0.2962 for solubility, r=0.474 for XlogP3, |r| = 0.403 for logP, and 0.0045508 for vapor pressure.
Graphical Analysis of Correlation Coefficients
The correlation between two or more sets of numbers or measures is shown mathematically in a graph. Graphs are useful and efficient tool for summarizing the results of the experiment and show the functional relationship between two experimental variables.
A Microsoft Excel worksheet's data shows graphically in an Excel graph. You can determine patterns, spot trends, compare data, and acquire ideas outside the boundaries of statistical data with Excel graphs.
Numerous graph and chart alternatives, such as bar graphs, line graphs, and pie charts, can be obtained in Excel. A continuous variable, such as time, temperature, or pressure, is represented by the X values in a line graph rather than in a scatter diagram. It graphs a set of related variables to show how Y shifts as a function of X. Line graphs typically use the Y-axis for the dependent variable and the X-axis for the independent variable.
All topological indices are labeled along the X-axis, and their respective correlation coefficients are shown along the Y-axis. Molar mass (MM), melting point (MP), XLOGP3, complexity (P), vapor pressure (VP), and solubility (S) are all shown in a scatterplot with many topological indices in Figure 4, and the comparison of all correlation values between properties and indices are graphically depicted in Figure 5. These graphs are generated by using the calculated values of all the correlation coefficients which are presented in Table 13.
Conclusion
In this work, the ten reducible TIs for drugs used to treat stroke disease were computed, and they were contrasted with a linear QSPR model along with seven physicochemical properties of the related drugs. The utilization of topological indices in combined with linear regression models enabled us to make predictions about the properties of drugs. This study has uncovered numerous interesting findings regarding the correlation coefficients among different indices.
In particular, the reducible first and second Zagreb index, the reducible Randic index, the reducible sum connectivity index, the reducible first hyper Zagreb index, and the first Gourava index show a strong correlation with the two specific properties such as molar mass and complexity at the range of 0.9.
The reducible second hyper Zagreb index demonstrates a strong positive correlation with molar mass at 0.8775 and with complexity at 0.8849.
The reducible Sigma index shows a strong positive correlation with molar mass at 0.9449 and with complexity at 0.8625. The reducible second Gourava index gives the best fit model with molar mass at 0.8301 and with complexity at 0.9147. Furthermore, it was discovered that only the reducible Forgotten index does not show a strong correlation with all the seven properties. Only two specific properties out of seven physicochemical properties such as molar mass and complexity is the best fit properties with all the defined ten reducible indices except Forgotten index. The pharmaceutical industry will be able to develop new drugs and identify preventative treatments for the aforementioned illness with the help of the data collected in this way.
These results are highly valuable for scientists investigating drug science in the pharmaceutical field, as they unveil an effective method to examine physicochemical features and identify new possibilities for treating different disorders.
Acknowledgements
None.
Conflict of Interest
The authors declare that there is no conflict of interest in this manuscript.
Data Availability
No data were used in this manuscript.
Founding statement
This research received no funding.
Orcid:
Samirah H. Alsulami*: https://orcid.org/0000-0001-7493-9609
------------------------------------------------------------------------------------------
How to cite this article: Samirah H. Alsulami*, A novel formation of molecular descriptors and quantitative structure-activity relationship analysis of thermodynamic aspects of stroke medicines. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(4), 428-446. Link: http://jmpcr.samipubco.com/article_185747.html
------------------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
