Document Type : Original Research Article
Authors
- Roihatul Mutiah 1
- Ahmad Ainur Roziqin 1
- Avin Ainur Fitrianingsih 2
- Ermin Rachmawati 2
- Imam Imam 3
- Rahmi Annisa 1
1 Department of Pharmacy, Faculty of Medical and Health Science, Universitas Islam Negeri Maulana Malik Ibrahim Malang, East Java, Indonesia
2 Department of Biomedical Sciences, Faculty of Medicine and Health Science, Universitas Islam Negeri Maulana Malik Ibrahim Malang, East Java, Indonesia
3 Indonesia National Agency of Drug and Food Control, Ambon Maluku, Indonesia
Abstract
This study aimed to develop an HPTLC-Densitometry method for identifying and determining the 1,4-naphthoquinone compound in ethanol and water extracts of Eleutherine palmifolia (EP). The method was validated for linearity, accuracy, precision, Limit of Detection (LOD), and Limit of Quantification (LOQ). The linearity is assessed to ensure a linear relationship between the instrument response and various analytical concentrations. Accuracy is measured by comparing the measurement results with reference values or true values. Precision is evaluated through repeated measurements on samples with the same concentration, both within (intra-assay) and between assays (inter-assay). The Limit of Detection (LOD) determines the lowest concentration that can be detected, while the Limit of Quantification (LOQ) determines the lowest concentration that can be measured and quantified accurately with a specific level of confidence. The linearity test yielded an r-value of 0.9976, with an LOD value of 163.6006 ppm and a LOQ value of 495.7595 ppm. The method's accuracy ranged from 80-120%, and the precision value from %RSD was 0.99%. The 1,4-naphthoquinone content in EP ethanol extract was found to be 0.318% (w/w), while in water extract it was 0.092% (w/w). The method meets standard validation requirements and is recommended for use in traditional medicine development due to its high concentration of 1,4-naphthoquinone.
Graphical Abstract
Keywords
Introduction
Eleutherine palmifolia (EP) is a plant reported to possess multiple therapeutic properties such as antioxidative, anticarcinogenic, and anti-inflammatory effects, antidiabetic, and antimicrobial activities [1-4]. The EP contains various substances including flavonoids (like quercetin, kaempferol, and apigenin), alkaloids (such as eleutherine and palmatine), polyphenols (including gallic acid and chlorogenic acid), naphthalenes (Eleuthroside A-D, Eleutherinol, B-sitosterol), and naphthoquinones (Eleutherine, Eleuthraquinone A-B, and 1,4-naphthoquinone). Among these, 1,4-naphthoquinone is a key component in EP and has a crucial impact on its therapeutic properties [5].
HPTLC-Densitometry, an approach employed for the measurement of quantity and quality control in pharmaceutical products, combines High-Performance Thin-Layer Chromatography (HPTLC) with densitometric measurement. This approach assists in establishing of specific compound content in samples by using a densitometer detector that measures the light intensity at compound spots on the chromatography plate [6].
The HPTLC-Densitometry method offers advantages in the determination of quantity and quality control of pharmaceutical products, such as relatively fast analysis speed, high sensitivity, effective compound separation, and the capability to analyze multiple samples on a single chromatography plate. In addition, it has strong validity and can be used for quantitative measurement of compound concentrations [7]. Determining the 1,4-naphthoquinone levels in EP extract through the HPTLC-Densitometry technique is essential, given that 1,4-naphthoquinone is a significant active ingredient with potential anticancer activity [8-9]. Therefore, its quantification is important for controlling the quality of EP extract as a pharmaceutical product.
The HPTLC Densitometry technique is employed to determine the level of 1,4-naphthoquinone in EP extracts with notable precision and sensitivity. Utilizing this technique, one can quantify the light absorption of 1,4-naphthoquinone spots, which is indicative of the compound's concentration within the sample. Verifying the amount of 1,4-naphthoquinone is crucial for confirming that EP extracts adhere to predefined quality benchmarks, thereby assuring the safety and efficacy of pharmaceuticals derived from these extracts. Consequently, the aim of this research is to employ the HPTLC Densitometry technique for quantifying the concentrations of 1,4-naphthoquinone in both ethanol and water extracts of EP. Furthermore, this study seeks to create a verified method for measuring 1,4-naphthoquinone using the HPTLC Densitometry approach.
Methodology
Standards and materials
The standards used in this study are 1,4-naphthoquinone obtained from Sigma Aldrich (Darmstadt, Germany).
Herbal material Eleutherine palmifolia was obtained from the South Malang area of East Java, Indonesia, in January 2022. The identification of this herbal plant has been carried out at the UPT Materia Medica Herbal Laboratory and documented with identification letter no. 074/212/102.20-a/2022.
Other materials used in this research include 96% ethanol, ethyl acetate p.a (Merck), hexane for analysis (Merck), absolute ethanol p.a (Merck), methanol for HPLC (Merck), deionized water, chloroform p.a (Merck), standard 1,4-naphthoquinone, and Eleutherine palmifolia bulbs.
Equipment
The instruments utilized in this study comprise a Developing chamber (Camag), Nanomat Contact Application (Camag), a device for analyzing moisture, TLC Visualizer (Camag), equipment for Ultrasonic Assisted Extraction, a Rotary Evaporator, a Freeze dryer, TLC Capillary Pipette 2µl (Camag), vortex mixer (Digisystem VM-2000), an analytical balance (Mettler Toledo AL-204).
Methods
Extraction using ultrasonic assisted extraction method
A total of 25 g of EP powder was subjected to extraction using 500 mL of 70% ethanol. solvent divided into three portions: 200 mL, 150 mL, and 150 mL in a sonicator bath at a frequency of 20 kHz for 2 minutes with three repetitions. After each extraction process, the mixture of EP powder and 70% ethanol was filtered using filter paper. The extract was concentrated using a rotary evaporator. After concentration, the filtrate was dehydrated in an oven at 50 °C for a duration of 24 hours [10].
Extraction using freeze drying method
Fresh EP bulbs were crushed using hot water as a solvent to dissolve all polar compounds. After the temperature dropped, the EP bulbs were filtered using filter paper aided by vacuum. Once the water filtrate was obtained, the liquid filtrate was then transferred to a freeze dryer. Inside the freeze dryer, before the drying process, the liquid filtrate was first frozen at -40 °C. In the frozen state, the liquid filtrate started to dry, facilitating the sublimation process (transition from solid (frozen) to gas) to occur. The final result of the extraction process using the freeze drying method yielded dried EP bulb extract [11].
Method validation
Linearity
Standard 1,4-naphthoquinone powder was measured and prepared in six concentrations ranging from 2.5 mg to 15 mg, increasing by 2.5 mg for each concentration, and then each amount was dissolved in 96% ethanol. Subsequently, this produced stock solutions with concentrations from 2500 ppm to 15000 ppm. These solutions were then applied to an HPTLC plate. Elution was then carried out in a mobile phase of chloroform: methanol (8:2 v/v), and the resulting spots were examined under UV light at λ 254 nm and 366 nm. Following this, the results were analyzed using a densitometer at a λ of 249 nm. The linear regression equation y = bx + a and the correlation coefficient (r) were determined from these results. [12-13].
Assessment of the limit of detection (LOD) and the limit of quantification (LOQ).
Dilution was carried out with 96% ethanol, creating solutions with varying concentrations 20 ppm-2500ppm. These solutions were then spotted onto an HPTLC plate. For the development of the HPTLC plate, the chosen mobile phase was chloroform:methanol (8:2 v/v).
The spots were analyzed using a densitometer at λ 249 nm. To determine the LOD (Limit of Detection) and LOQ (Limit of Quantification) in this study, Equations (1) and (2) were used as follows [14]:
LOD (limit of detection)
LOD: (3.3 X σ): S (1)
LOQ (Limit of quantification)
LOQ : (10 X σ) :S (2)
Accuracy
Standard solutions were prepared with concentrations of 9 mg (90%), 10 mg (100%), and 11 mg (110%). These solutions were then diluted with 1 mL of 96% ethanol. The EP extract was diluted to a concentration of 20000 ppm until it reached a volume of 1 mL. Each of these solutions was then applied to an HPTLC plate, repeated three times to produce a total of nine spots. Subsequently, analysis was carried out using a densitometer, and the recovery percentage was calculated [14,15].
Precision
A test solution having a 20000 ppm concentration was administered onto an HPTLC plate on six occasions. Subsequently, the plate underwent densitometer scanning to ascertain the relative standard deviation (RSD%) value. The results are deemed satisfactory if the measured RSD% is less than 5% [14,16].
Determination of compound concentration
The concentration of the compound 1.4 naphthoquinone was determined from the linear regression equation in the linearity test by inputting the obtained area percentage [17].
Results and discussion
Linearity
The linearity test, conducted subsequent to the selection of the mobile phase, is designed to evaluate an analytical method's capacity to produce consistent results relative to the analyte concentration within the sample (Kounnoun et al., 2020).
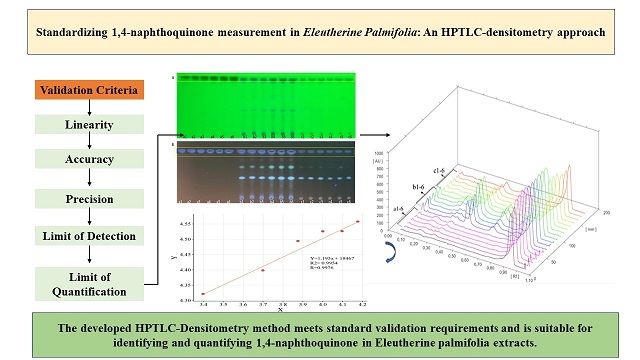
During the method validation for linearity determination, the derived linear formula used was y = 1.195x + 18467, exhibiting a coefficient of determination (R-squared) of 0.9954, and a correlation coefficient (r) of 0.9976 (Figure 2). These figures reflect the strength and degree of the relationship and fit between the concentration (x-variable) and the densitometric intensity (y-variable).
An R-squared value of 0.9954 suggests that around 99.54% of the variance in the densitometric intensity is accounted for by changes in concentration, indicating a robust correlation between the measured concentrations and the corresponding densitometric intensities [6].
Additionally, the correlation coefficient (r) of 0.9976 signifies an exceedingly strong linear correlation between the concentration and the densitometric intensity. An r-value nearing 1 denotes a positive and pronounced linear association between these two variables [14,18].
Given the high R-squared and r-values, the linearity equation is deemed valid and precise for correlating concentration with densitometric intensity. This affirms the suitability of the adopted method for accurate quantification of 1,4-naphthoquinone levels in samples via the HPTLC densitometry technique.
Detection limit (LOD) and quantification limit (LOQ)
In the method validation for determining the level of 1.4-naphthoquinone in EP extract, the LOD value of 163.6006ppm and LOQ value of 495.7595 ppm were obtained (Table 1). LOD represents the sensitivity of the analytical method to detect the presence of 1.4-naphthoquinone in a sample [16]. A LOD value of 163.6006 ppm indicates that the method can detect the presence of 1.4-naphthoquinone in EP extract at concentrations as low as 163.6006 ppm [14]. With a low LOD, the method exhibits a sensitive detection capability for 1.4-naphthoquinone.
LOQ represents the sensitivity of the analytical method to measure and determine the level of 1.4-naphthoquinone in a sample with adequate accuracy. A LOQ value of 495.7595 ppm indicates that the method can accurately measure the level of 1.4-naphthoquinone in EP extract down to a minimum concentration of 495.7595 ppm. A low LOQ signifies that the method has good capability to measure the level of 1.4-naphthoquinone in a sample with sufficient precision [19].
Accuracy and precision
Accuracy and precision are two critical aspects of method validation that guarantee the dependability and accuracy of analytical outcomes. Accuracy describes the closeness of measured values to the actual or true values. In method validation, accuracy is determined by comparing the method's results to those obtained from a known reference standard or a previously validated method. A method that exhibits a high level of accuracy yields results that align closely with the true value. Accuracy is commonly assessed by calculating the percent recovery or by comparing the results against a known standard [20] .
Precision, in contrast, refers to the level of repeatability or consistency of the results. It evaluates the method's stability and dependability under varying conditions or when used by different analysts. Precision is typically measured by calculating the relative standard deviation (RSD) or by executing multiple measurements and examining the variation among them. A low RSD signifies high precision, indicating that the method produces consistent and reliable results [13,21].
The HPTLC densitogram presented in Figure 3 offers a comprehensive view of the densitogram spectrum. The standard 1,4-naphthoquinone is displayed across tracks a1-a6, the ethanol extract in tracks b1-b6, and the water extract in tracks c1-c6. The densitogram shows the presence of 1,4-naphthoquinone in both extracts, with noticeable differences in the compound profiles of each extract. Furthermore, the data regarding the Retention Factor (Rf) and the percentage area of each sample, as detailed in Table 2, indicate that both extracts contain 1,4-naphthoquinone with an identical Rf value of 0.96. This substantiates the presence of 1,4-naphthoquinone in both extracts of EP.
In the process of procedure verification for measuring the quantity of 1,4-naphthoquinone in EP extract, a % recovery of 101.89% was achieved, which is within the acceptable standard range of 80% to 120%. This outcome demonstrates that the technique is precise and dependable for measuring the level of 1,4-naphthoquinone in the EP extract. A % recovery exceeding 100% indicates commendable accuracy, as it implies the method recovers slightly more than the anticipated amount of the analyte. Moreover, the %RSD (Relative Standard Deviation) achieved was 0.996%, which falls below the acceptable threshold of less than 2% for the standard range. A low %RSD reflects a high degree of precision of the method. The minimal variation among replicate measurements underscores the method's consistency and repeatability [13,17].
In conclusion, the % recovery of 101.89% confirms the method's accuracy, and the %RSD of 0.996% affirms its precision in measuring the amount of 1,4-naphthoquinone (Table 3). These findings validate the method's reliability and its applicability for quantitative analysis in this context.
Identification of 1,4-naphthoquinone
The identification of the compound 1,4-naphthoquinone in the ethanol and water extracts of EP revealed the same Rf value, confirming the presence of the same )compound (Figure 4). However, the quantification of 1,4-naphthoquinone in the ethanol extract of EP showed a higher percentage of 0.318% compared to 0.092% in the water extract (Table 4).
This variance in percentages suggests that the ethanol extract has a higher level of 1,4-naphthoquinone than the water extract. This increased percentage implies that the ethanol extraction process is more effective in extracting and preserving 1,4-naphthoquinone from the sample. It should be noted that these quantification results are in relation to the concentration of 1,4-naphthoquinone within the respective extracts, and the elevated percentage in the ethanol extract indicates a greater presence of the compound in that specific extract [22].
Conclusion
The method validation results for determining the amount of 1,4-naphthoquinone in ethanol and water extracts. of EP using HPTLC-densitometry provided the following insights: The linearity test yielded a correlation coefficient (r) of 0.9976, which indicates a strong linear relationship. Precision was ascertained with a Limit of Detection (LOD) at 163.6006 ppm and a Limit of Quantification (LOQ) at 495.7595 ppm. Accuracy was confirmed by a recovery percentage within the acceptable range of 80-120%, and precision was reflected by a %RSD of 0.99%. Moreover, the concentration of 1,4-naphthoquinone was determined to be 0.318% (w/w) in the ethanol extract and 0.092% (w/w) in the water extract of EP. These findings verify that the data meets the criteria for method validation. Concerning the ethanol extract contains a higher level of 1,4-naphthoquinone than the water extract, it is advised to use the ethanol extract in creating traditional medicinal formulations because of its higher concentration of the active ingredient.
Acknowledgements
The authors would like to thank the National Research and Innovation Agency for the funding provided for this research, allowing it to proceed smoothly
Funding
This research was funded by the National Research and Innovation Agency (BRIN) based on the decision of the Deputy for Research and Innovation Facilitation of the National Research and Innovation Agency number 37/II.7/HK/2023 in the Research and Innovation Program for Advanced Indonesia wave 4.
Conflict of Interest
The authors declare that they have no conflict of interest in this study.
Data Availability
All data produced and examined are incorporated in this research article.
Orcid:
Roihatul Mutiah: https://orcid.org/0000-0002-8196-9029
Ahmad Ainur Roziqin: https://orcid.org/0009-0008-9954-0491
Avin Ainur Fitrianingsih: https://orcid.org/0000-0002-3571-114X
Ermin Rachmawati: https://orcid.org/0000-0003-1045-7066
Imam Taufik: https://orcid.org/0000-0003-4959-7488
Rahmi Annisa*: https://orcid.org/0000-0001-7536-5213
--------------------------------------------------------------------------------
How to cite this article: Roihatul Mutiah, Ahmad Ainur Roziqin, Avin Ainur Fitrianingsih, Ermin Rachmawati, Imam Taufik, Rahmi Annisa*, Standardizing 1,4-naphthoquinone measurement in Eleutherine Palmifolia: An hptlc-densitometry approach. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(5), 481-491. Link: http://jmpcr.samipubco.com/article_186480.html
---------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
