Document Type : Original Research Article
Authors
- Galih Sampoerno 1
- Sukaton Sukaton 1
- Ekarista Lussiana Ferdinandus 2
- Najwa Nadaa Firdaus 2
- Rista Dalila Damayanti 2
1 Department of Conservative Dentistry, Faculty of Dental Medicine, Airlangga University, Surabaya, Indonesia
2 Conservative Dentistry Specialist Program, Faculty of Dental Medicine, Airlangga University, Surabaya, Indonesia
Abstract
Microorganism contamination can induce dental pulp inflammation and generate pain. In this process, complex communication occurs between the immune and nervous system components until pain perception occurs. An improved understanding of the interactions involved in the dental pulp inflammatory pain process can be the basis for developing potential therapeutic agents. The current study aims to analyze the inflammatory pain process that follows the application of Porphyromonas gingivalis lipopolysaccharide (LPS) to dental pulp tissue by examining the expression of calcitonin-gene-related peptide (CGRP) in neurons (neuron-CGRP) and macrophages (macrophage-CGRP) as well as the expression of NaV 1.8 in neurons (neuron-NaV 1.8). This experimental laboratory study utilized 32 Spraque Dawley rats, divided into two groups. In the mandibular incisors, the control group only had access openings, while the treatment group had access openings and P. gingivalis LPS injections. Each group was terminated after 48 hours. Then slide preparations were made, and immunohistochemical staining was done to observe the expressions of neuron-CGRP, macrophage-CGRP, and neuron-NaV 1.8 using a light microscope. The LPS administration induced a significant increase in the expression of neuron-CGRP, macrophage-CGRP in macrophages, and neuron-NaV 1.8. There was no significant difference between the neuron-CGRP and macrophage-CGRP expressions. The neuron-CGRP is significantly and positively correlated with neuron-NaV 1.8. The microbial contamination via P. gingivalis LPS application to dental pulp tissue can increase the expression level of CGRP in both neurons and macrophages. However, only neuron-CGRP has been proven to significantly cause an increase in neuron-NaV 1.8 expression.
Graphical Abstract
Keywords
Introduction
Many studies have been conducted on the inflammatory response in the dental pulp, which is a dynamic and complicated response involving neuronal, vascular, and immunologic responses to different damages such as dental trauma, caries, periodontal disease, and operative dentistry procedures. However, the invasion of bacteria and its elements is the primary cause of pulp inflammation [1]. A diverse microorganism habitat mainly consists of gram-negative bacteria, particularly obligate anaerobic species, is a characteristic of pulp tissue infections. Porphyromonas gingivalis was shown to be highly prevalent (58.8%) in the bacteria linked to primary endodontic infections and irreversible pulpitis, according to research by Zargar et al. (2020) [2].
Gram-negative bacteria have lipopolysaccharide (LPS) in their cell walls, which is a potent endotoxin that triggers an inflammatory reaction. LPS can therefore directly affect the host's immune system and vulnerability to disease [3]. LPS stimulates several downstream signaling pathways by attaching to the cell's Toll-Like Receptors (TLRs), which results in the production of inflammatory mediators. Bacterial lipopolysaccharide (LPS) is a crucial starter in the pathophysiology of pulpitis. It enters the afflicted dental pulp tissue and stimulates the significant production of inflammatory mediators, which in turn sets off the pulp's inflammatory response [4].
The 37-amino acid peptide known as calcitonin gene-related peptide (CGRP), which was initially discovered in 1982, is created when the calcitonin gene (CT/CALCA) is spliced alternatively. The peripheral and central sensory nerve systems are where CGRP is mostly distributed. The most typical associations of CGRP are with sparsely myelinated Aδ nerve fibers and small-diameter unmyelinated sensory C nerve fibers [5]. The G protein-coupled receptor (GPCR), also referred to as the calcitonin receptor-like receptor (CALCLR/CLR), is responsible for mediating its biological activity. It forms a receptor complex with accessory proteins, namely receptor component protein (RCP) and receptor activity-modifying protein (RAMP), to modify the transduction of signals from ligand binding and its selectivity [6]. Depending on where it is expressed, CGRP can have a variety of physiological functions, including as nociception, metabolism, vasodilation, and inflammation [7]. The sensory nerve fibers that innervate the tooth pulp include CGRP, one of the numerous neuropeptides in the trigeminovascular system. By boosting the production of inflammatory mediators, inducing vasodilation, influencing pulp blood flow and collateral circulation, and even inducing vascular collapse, CGRP and SP work in concert to alleviate the dental pulp inflammatory process [8]. Depending on the level of each stimulus, CGRP may also be associated with the mechanism of orofacial inflammatory pain brought on by orthodontic movement forces, occlusal trauma stimulation, or their combination [9]. It is possible that CGRP regulates odontoblast development and function during dentin bridge formation and wound healing [10]. When compared to healthy pulps, pulps with irreversible pulpitis have noticeably greater levels of CGRP expression [11,12].
It is commonly known that neuronal cells generate the neuropeptide CGRP. Nevertheless, CGRP may also be generated by non-neuronal cells, as many investigations have demonstrated. Emerging evidence indicates that immune cells and nerve cells can both emit neuronal peptides that regulate the immune system's reaction to external injuries [13]. It has been demonstrated that immune cells such B lymphocytes [14,15] and monocytes [16] generate CGRP, demonstrating their function in regulating inflammatory and immunological responses. Research conducted by Ma et al. [17] shown that LPS endotoxin at dosages of 0.1 and 1 μg/mL escalated the amount of CGRP in peritoneal macrophages of Spraque-Dawley rats. In the meanwhile, Ma's 2010 study [18] shown that RAW264.7 macrophage cells' release of CGRP was markedly enhanced by LPS induction at doses of 0.1 and 1 μg/mL for 6, 12, 24, (the maximum release), and 48 hours.
Inflammatory pain and peripheral sensitivity in the tooth pulp are significantly heightened by CGRP [19]. In the meanwhile, macrophages play a part in inflammatory and immunological responses and are a significant source of CGRP [13]. Nevertheless, only a few studies has been done on the CGRP that macrophage cells create. In fact, no studies have been done to discover CGRP expression in tooth pulp tissue macrophages. The expression of CGRP by macrophages in dental pulp tissue in response to P. gingivalis LPS administration, as well as its possible role in dental pulp inflammation and pain mechanism, were therefore among the goals of this current work.
One of the clinically utilized signs and symptoms to diagnose pulp inflammation is pain, which is one of the pathophysiological changes brought on by pulp inflammation. Transduction, transmission, modulation, and perception are the four primary mechanisms involved in pain perception. Afferent nerve terminals convert inputs into nociceptive impulses through a process called transduction [20]. One of the main processes behind dental pain during inflammation is the reduction in the threshold for ectopic action potentials arising from the nociceptive pathway, which increases the sensitivity of primary sensory neurons and generates spontaneous pain. Increased expression of voltage-gated sodium channels (VGSC) in primary afferent neurons lowers the action potential threshold, which in turn causes an increase in action potential [21,22].
Transmembrane ion channels known as voltage-gated sodium channels (VGSCs) contribute in the initiation and development of action potentials as well as the excitability of the cell membrane [21,22]. NaV 1.8. is one of the tetrodotoxin-resistant (TTX-R) sodium channels that is primarily located in the unmyelinated C nerve fiber, the major group of sensory nerve fibers that innervate the dental pulp tissue. Nav1.8 is an isoform that is more upregulated in diseased teeth compared to normal pulp. Its location along the nerve axon promotes faster conduction of impulses from the terminal to the central terminal, thereby mediating the spontaneous and sharp pain that is characteristic of pulpitis. It is assumed that NaV 1.8 has an essential function in regulating the pain features of irreversible pulpitis since this channel can form clusters in myelinated segments of unmyelinated axons [23]. Studies demonstrated a considerable increase in NaV 1.8 expression in primary tooth pulp that was inflamed [24,25].
The aforementioned explanation gave rise to the idea of researching the process of inflammatory pain in the dental pulp that follows the application of P. gingivalis LPS to dental pulp tissue through the expression of CGRP in neurons (neuron-CGRP) and macrophages (macrophage-CGRP) and the expression of NaV 1.8 in neurons (neuron-NaV 1.8). A deeper comprehension of the immune system's and nervous system's interactions throughout the dental pulp inflammatory pain process may serve as the foundation for the identification of viable targets for dental pulp inflammatory pain treatment.
Materials and methods
This was an experimental laboratory study utilizing thirty-two male Spraque Dawleys that matched the inclusion criteria (full eruption of mandibular incisors, age of twenty weeks, weight as a whole of 425-450 grams, and fair health). The Federer formula was used to determine the sample size. The rats were assigned randomly into the control group and the LPS application group. The unit of analysis was the mandibular incisors. The Health Research Ethical Clearance Commission, Faculty of Dental Medicine, Airlangga University, examined and authorized every technique used in this study (Certificate Number: 882/HRECC. FODM/ XII/2022).
To sedate each sample in all treatment group, an intraperitoneal injection of sterile Phosphate Buffer Saline (PBS) diluted with 80 mg/kg of ketamine and 10 mg/kg of xylazine was given. Using a high-speed handpiece and a fissure bur, the pulp chamber was opened by creating a flat plane at the level of the interdental papillae (3 mm), and then perforation of the pulp chamber was done with a round bur. Following this procedure, the pulp chamber was injected with 10 µl Porphyromonas gingivalis lipopolysaccharide (Ultrapure lipopolysaccharide from Porphyromonas gingivalis–TLR4 ligand, Catalog # tlrl-ppglps. Version #14F18-MM. InvivoGen, San Diego, CA USA) and sealed with glass ionomer cement. This was done in the LPS application group. In contrast, the control group's cavity was sealed with glass ionomer cement without the LPS injection. A ketamine and xylazine overdose injection was used to terminate all samples 48 hours later. The mandibles were then isolated, fixated, and left in 10% buffered formalin for 24 hours. After being decalcified for 30 days using 4% ethylene diamine tetraacetic acid (EDTA), the tissues are made into a paraffin block. Using a microtome, tissues in paraffin blocks were cut into a thickness of 4 μm, then put on polysine slides and heated to 56-58 °C for an entire night. The slides were then immersed in 3% hydrogen peroxide for 30 minutes at room temperature to eliminate the endogenous peroxide activity. After deparaffinizing the 4 µm periapical tissue slices in xylol, they were rehydrated using a graded alcohol and water (xylol for 20 minutes, 100% alcohol for 5 minutes, 95% alcohol for 5 minutes, and 70% alcohol for 5 minutes). Afterwards, they were rinsed for 5 minutes under running water.
Using immunohistochemical staining, the expression of neuron-CGRP, machrophage-CGRP and neuron-NaV 1.8 were examined. The antibody of CGRP and NaV 1.8 (Anti-rat monoclonal antibody, Santa Cruz Biotechnology Inc., Texas, USA) were used. A light microscope (Nikon E100 LED binocular microscope, Nikon, New York, USA) with a 1000x magnification was used to examine the preparations.
Results
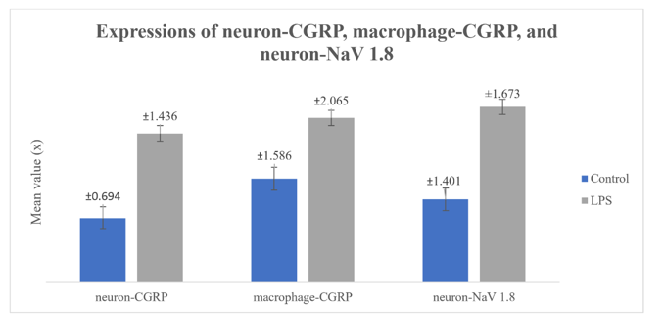
Figures 1, 2, and 3 demonsrtate the histological observation results for the expression of neuron-CGRP (Figure 1 (A-B)), macrophage-CGRP (Figure 2 (A-B)), and neuron-NaV 1.8 (Figure 3 (A-B)). The LPS application groups showed a higher expression compared to the control groups.
The data obtained from the study on dental pulp inflammation following lipopolysaccharide administration based on the expression of neuron-CGRP, macrophage-CGRP, and neuron-NaV 1.8 are presented in Table 1. The Shapiro-Wilk and Levene tests were performed to look at the data distribution and homogeneity for each group before the difference test (Independent T-test) was applied to each group. The data distribution for each treatment group is normal (p > 0.05) and the variance is homogeneous (p > 0.05).
As shown in Table 1 and Figure 4, LPS application on dental pulp tissue group showed a significantly higher neuron-CGRP, macrophage-CGRP and neuron-NaV 1.8 than in control group (p = 0,001).
Afterwars, the CGRP expression level following LPS application was compared between neuron-CGRP and macrophage-CGRP. The mean value of macrophage-CGRP expressions seems higher than neuron-CGRP expression. However, as listed in Table 2, the Independent T-test revealed that there was no significant difference (p = 0.328).
The correlation between neuron-CGRP, macrophage-CGRP, and neuron-NaV 1.8 was ascertained using the Stepwise Regression Analysis Test. The results, as provided in Table 3, showed that there was a positive and significant correlation between the neuron-CGRP and neuron-NaV 1.8 (p = 0.001). The correlation between macrophage-CGRP and neuron-NaV 1.8 has a value of p = 0.556 (p > 0.05), which means there is no significant relationship between macrophage-CGRP and neuron-NaV 1.8 expressions.
.png) |
Discussion
Increased CGRP expression in neurons and macrophages after LPS application The present investigation validated the noteworthy elevation in CGRP expression in neurons subsequent to the LPS administration. The increase in CGRP expression is due to the recognition of LPS by its receptor, TLR4, on the neurons surface. Following its recognition of LPS, TLR4 will start the myeloid differentiation protein 88 (Myd 88) pathway, turn on protein kinase C (pKC), and then subsequently bind to transient receptor potential vanilloid 1 (TRPV1). After that TRPV1 will control the production and discharge of CGRP, which will raise the expression of neuron-CGRP [13]. The findings of this investigation align with several previous studies that demonstrate injecting LPS intraperitoneally into male mice causes intracellular signaling by binding to the TLR4 membrane receptor, which subsequently modifies the release of CGRP [26]. Increased CGRP expression also occurs when P.gingivalis LPS is applied, followed by extirpation of dental pulp tissue, through TLR4 activation [27]. In Kaewpitak et al.'s research [28], it was demonstrated that P. gingivalis LPS rapidly stimulates trigeminal sensory neurons through the TLR4, TRPA1, and TRPV1 receptors, leading to enhanced production and release of CGRP. In comparison to teeth with healthy pulp, teeth with irreversible pulpitis showed a substantial increase in CGRP expression [11-12,29].
The expression of CGRP by dental pulp macrophages following P. gingivalis LPS injection has been successfully proven by this study. When LPS binds to its receptor (TLR4) distributed on the macrophages surface, the Myd88 pathway is triggered, which in turn causes intracellular signal transduction in macrophages. The next step involves the activation of interleukin-1 receptor-associated kinase (IRAK) leading to the recruitment of tumor necrosis factor receptor-associated factor 6 (TRAF-6), as well as turning on the inhibitor of IκB kinase (IKK). Physiologically, IKK will attach to I-κB, activate the cytoplasmic inactive nuclear transcription factor kappa-B (NF-κB), and release NF-κB into the nucleus of the cell. NF-κB is a protein complex that regulates the transcription of deoxyribo nucleic acid (DNA) in the cell nucleus enabling the transcription and secretion of tumor necrosis factor alpha (TNF-α). By connecting to the tumor necrosis factor alpha receptor (TNFR) on the cell surface, the released TNF-α can re-enter the macrophage and activate tumor necrosis factor receptor associated factor-2 (TRAF-2). Thereby, it triggers mitogen activated protein kinase/extracellular regulated kinase kinase (MEK) to phosphorylate mitogen activated protein kinase (MAPK) and initiates the secretion and release of CGRP, resulting in an increase in macrophage-expressed CGRP.
The macrophage-CGRP expressions shown in this current investigation verify the statement that CGRP can be secreted by non-neuronal cells. This is consistent with the finding made by Assas [13] on mounting data indicating the potential role that neural peptides produced by both neuronal and non-neuronal cells play in controlling the immune system's reaction to external injuries. A few recent studies that corroborate this claim are those by Yin et al. [30], which showed elevated CGRP expression in Aspergillus fumigatus-induced mouse corneal macrophages, and Duan's [31], which demonstrated increased CGRP mRNA and protein in mouse macrophages (RAW 264.7) induced by 1 μg/mL LPS.
Table 1 indicates that the average expression level of macrophage-CGRP appears to be higher (6.56) than that of neuron-CGRP (5.94). Nevertheless, there was no noteworthy distinction in the expression of neuron-CGRP or macrophage-CGRP following LPS treatment, based on the result of Independent T-test (Table 3). The presence of migratory macrophages, which are drawn from blood vessels to the site of inflammation and express CGRP, is likely responsible for the greater level of macrophage-CGRP. Acute phase proteins, chemokines, and cytokines are released during the acute phase of the inflammatory process following an injury. These mediators provoke neutrophils and macrophages to migrate over the blood circulation to the actual location of inflammation [32]. According to Tajima et al. [33], LPS stimulation of E. coli promotes macrophage migration through activation of Prostaglandin D2 and Prostaglandin E2. In conjunction with pulp tissue-resident macrophages, located in cell-rich zone, these incoming macrophages then express CGRP according to the signalling pathway described previously. Hence, the CGRP expression in macrophages appears to be greater in quantity than in neurons. Increased NaV 1.8 expression in neurons after LPS application
The level of neuron - NaV 1.8 expression increased significantly after LPS administration. The next sections outline a few possible paths that might lead to this. CGRP, whether expressed by neurons or macrophages, will then be identified by its receptor on the surface of neurons, i.e. the calcitonin receptor-like receptor (CALCLR). The introduction of CGRP by CALCLR in neurons will activate phospholipase C (PLC) to create diacylglycerol (DAG). Furthermore, DAG will also activate protein kinase C (pKC) and phosphorylate sodium channels to regulate their function, leading to increased NaV 1.8 expression. On the other hand, CGRP binding to CALCLR on the neurons surface also activates adenylyl cyclase (AC), which in turn produces cyclic adenosine monophosphate (cAMP). Next, cAMP will activate cAMP-dependent protein kinase (pKA) and phosphorylate sodium channels to regulate their function, resulting in increased NaV 1.8 expression. This explanation is in line with Natura et al.'s [34] study, which showed that CGRP elevates TTX-R Na+ currents in a subset of DRG neurons and is adequate to generate action potentials.
On another pathway, macrophage-produced TNFα binds to neuronal surface TNFR, activating tumor necrosis factor receptor-associated factor-2 (TRAF-2) and then triggering mitogen-activated protein kinase / extracellular-regulated kinase kinase (MEK) to phosphorylate the protein 38 mitogen-activated protein kinase (p38 MAPK), thereafter modulating sodium channels to regulate their function. Ultimately, this results in increased expression of neuron-NaV 1.8.
The elevated expression of neuron-NaV 1.8 in the dental pulp seen in this study is consistent with the findings of several other studies. The NaV 1.8 function in the process of pulp inflammatory pain was demonstrated by research by Renton et al. [35], which revealed an increase in NaV 1.8 expression in the pulp tissue group of patients experiencing tooth pain as a result of the caries process. Research by Suwanchai et al. [24] demonstrated that inflamed primary tooth pulp had a considerable increase in NaV 1.8 expression. Patients with lingual nerve neuroma who complained of pain were also shown considerably greater expression of NaV 1.8, according to the research by Bird et al. [36]. There was also a positive association between the patient's VAS score and NaV 1.8 expression. These researchers suggested NaV 1.8 as a potential target for pain therapy. Correlation between CGRP expression in neurons and macrophages with NaV 1.8 expression in neurons after LPS application
The results of pathway analysis, as summarized in Table 4, statistically prove a positive and significant correlation between neuron-CGRP and neuron-NaV 1.8. It means that the higher the neuron-CGRP expression, the higher the expression of neuron-NaV 1.8. This result proves that microbial injury from P. gingivalis LPS can trigger inflammatory pain transduction in the dental pulp by elevating the production of neuron-CGRP, which subsequently amplifies the expression level of neuron-NaV 1.8. This, in turn, will generate action potential. Further research is needed to develop neuron-CGRP and NaV 1.8 as potential therapeutic targets for dental pulp inflammatory pain therapy.
On the other hand, although having a greater average expression following LPS administration, pathway analysis revealed statistically that macrophage-CGRP did not have a significant association with neuron-NaV 1.8 expression. The reason for this might be that neuron-CGRP and mecrophage-CGRP are distinct isoforms that play different function. CGRP exists in two isoforms: αCGRP and βCGRP. With just three distinct amino acids, the two share 94% of their structural similarities. Both, nevertheless, are distributed in different locations. Whereas αCGRP is mainly found in the peripheral and central nervous systems, particularly in the dorsal root ganglion cell bodies, βCGRP is mostly found in the pituitary gland, intestine, and immunological cells such as T cells [37]. Research by Xing et al. [38] found that only βCGRP mRNA is expressed by mouse lymphocytes. Wang et al.'s research [15] also showed that human lymphocyte cells are capable of producing βCGRP. In mouse and human keratinocyte cultures, βCGRP mRNA is expressed greater than αCGRP, according to Hou et al. [39]. According to our estimation, it seems probable that following the injection of P. gingivalis LPS in the dental pulp tissue, the neurons will express more of the isoform αCGRP, whereas the macrophages will express more of the isoform βCGRP. Further examination is required to determine the CGRP isoform produced by nerve cells and macrophage cells in dental pulp tissue.
The role of CGRP released by non-neuronal cells is not yet clearly known, as is the function of CGRP expressed by dental pulp tissue macrophages. Some of these studies attempt to elucidate the function of non-neuronal CGRPP. Xing et al.'s study [38] shown that in rat cells, βCGRP mRNA functions as a negative modulator-feedback loop in response to immunological stimuli. According to research by Wang et al. [15], human lymphocyte cells' βCGRP is involved in limiting the growth and adjusting the functionality of human T-lymphocyte cells. According to research by Hou et al. [39], βCGRP mRNA in rat and human keratinocytes contributes differentially to pain situations and homeostasis maintenance. According to Hu et al.'s review [40], CGRP from non-neural sources is critical for regulating a number of physiological and pathological processes. It does this through a variety of channels, including autocrine/paracrine mechanisms. To fully understand how macrophage-CGRP contributes to the inflammatory process of tooth pulp tissue, more study is required.
Conclusion
The current study proved that microbial contamination via P. gingivalis LPS application can induce pain transduction, which is characterized by an increase in the expression of neuron-CGRP and macrophage-CGRP as well as neuron-NaV 1.8. Nevertheless, it has been demonstrated that only neuron-CGRP may positively and significantly elevate the expression of neuron-NaV 1.8, which in turn generates potential action that commences the pain transduction process.
Acknowledgements
The authors would like to thank the faculty of dental medicine Airlangga University for facilitating this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Galih Sampoerno: conduct research and owner of the frame work
Sukaton: manuscript editing
Ekarista Lussiana Ferdinandus: conduct research and manuscript editing
Rista Dalila Damayanti: design research methodology
Najwa Nadaa Firdaus: Statistic analysis
Conflict of Interest
The authors declare that there are no conflicts of interest.
Orcid:
Galih Sampoerno: https://orcid.org/0000-0003-1437-3185
Ekarista Lussiana Ferdinandus: https://orcid.org/0009-0000-4057-0049
-----------------------------------------------------------------------------------
How to cite this article: Galih Sampoerno, Sukaton Sukaton, Ekarista Lussiana Ferdinandus, Najwa Nadaa Firdaus, Rista Dalila Damayanti, Expression of CGRP and NaV 1.8 in neurons and macrophages after p.gingivalis lipopolysaccharide aplication on dental pulp tissue. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(5), 558-570. Link: http://jmpcr.samipubco.com/article_187568.html
-----------------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
[1] (a) K.M. Galler, M. Weber, Y. Korkmaz, M. Widbiller, M. Feuerer, Inflammatory response mechanisms of the dentine pulp complex and the periapical tissues, International Journal of Molecular Sciences, 2021, 22, 1480. [Crossref], [Google Scholar], [Publisher], (b) A. Salh, M.H. Risan, Jasim, Biochemical characteristics and antibiotics susceptibility of streptococcus mutans isolates from dental caries in baghdad city, International Journal of Advanced Biological and Biomedical Research, 2022, 10, 32-43. [Crossref], [Google Scholar], [Publisher], (c) A. Amini, H. Shahpoori Arani, M. Milani Fard, Medical tourism industry: A systematic review on its principles, sequels, and ethical issues, Eurasian Journal of Science and Technology, 2022, 2, 139-151. [Crossref], [Google Scholar], [Publisher], (d) E. Erdag, M. Kucuk, U. Aksoy, N. Abacioglu, A.O. Sehirli, Docking study of ligands targeting nlrp3 inflammatory pathway for endodontic diseases, Chemical Methodologies, 2023, 7, 200-210. [Crossref], [Google Scholar], [Publisher], (e) S. Korbag, I. Korbag, A short review on effects of bisphenol a and its analogues on endocrine system, J. Chem. Rev, 2023, 5, 380-393. [Crossref], [Google Scholar], [Publisher]
[2] N. Zargar, H. Ashraf, S.M.A. Marashi, M. Sabeti, A. Aziz, Identification of microorganisms in irreversible pulpitis and primary endodontic infections with respect to clinical and radiographic findings, Clin. Oral Investig., 2020, 24, 2099-2108. [Google Scholar], [Publisher]
[3] L. Mazgaeen, P. Gurung, Recent advances in lipopolysaccharide recognition systems, International Journal of Molecular Sciences, 2020, 21, 379. [Crossref], [Google Scholar], [Publisher]
[4] C . Lan, S. Chen, S. Jiang, H. Lei, Z. Cai, X. Huang, Different expression patterns of inflammatory cytokines induced by lipopolysaccharides from Escherichia coli or Porphyromonas gingivalis in human dental pulp stem cells, BMC Oral Health, 2022, 22, 121. [Crossref], [Google Scholar], [Publisher]
[5] K. Warfvinge, L. Edvinsson, Distribution of CGRP and CGRP receptor components in the rat brain, Cephalalgia, 2019, 39, 342-353 [Crossref], [Google Scholar], [Publisher]
[6] L. Edvinsson, A.S. Grell, K. Warfvinge, Expression of the CGRP Family of Neuropeptides and their Receptors in the Trigeminal Ganglion, Journal of Molecular Neuroscience, 2020, 70, 930-944 [Crossref], [Google Scholar], [Publisher]
[7] A. Bonura, N. Brunelli, M. Marcosano, G. Iaccarino, L. Fofi, F. Vernieri, C. Altamura, Calcitonin gene-related peptide systemic effects: embracing the complexity of its biological roles—a narrative review, International Journal of Molecular Sciences, 2023, 24, 13979 [Crossref], [Google Scholar], [Publisher]
[8] A. Heidari, M. Shahrabi, M.S. Shahrabi, M. Ghandehari, P. Rahbar, Comparison of the level of substance P and neurokinin A in gingival crevicular fluid of sound and symptomatic carious primary teeth by ELISA, Journal of Dentistry, Tehran University of Medical Sciences, 2017, 14, 173-179 [Google Scholar], [Publisher]
[9] H. Liang, H. Hu, D. Shan, J. Lyu, X. Yan, Y. Wang, F. Jian, X. Li, W. Lai, H. Long, CGRP modulates orofacial pain through mediating neuron-glia crosstalk, Journal of Dental Research, 2021, 100, 98–105 [Crossref], [Google Scholar], [Publisher]
[10] M. Zhang, H. Fukuyama, J. Zhang, T. Tanaka, Immunoelectron microscopic study of cgrp-immunoreactive nerve terminals in wound healing and dentin bridge formation after pulpotomy in rat molar, Acta Histochem, 2003, 36, 43-49. [Crossref], [Google Scholar], [Publisher]
[11] M. Sattari, M.A. Mozayeni, A. Matloob, M. Mozayeni, H.H. Javaheri, Substance P and CGRP expression in dental pulps with irreversible pulpitis, Aust Endod J, 2010, 36, 59–63. [Crossref], [Google Scholar], [Publisher]
[12] J. Caviedes-Bucheli, C. Camargo-Beltrán, A.M. Gómez-la-Rotta, S.C. Moreno, G.C. Abello, J.M. González-Escobar, Expression of calcitonin gene-related peptide (CGRP) in irreversible acute pulpitis, Journal of Endodontics, 2004, 30, 201-204. [Crossref], [Google Scholar], [Publisher]
[13] a) B.M. Assas, J.A. Miyan, J.L. Pennock, Cross-talk between neural and immune receptors provides a potential mechanism of homeostatic regulation in the gut mucosa, Mucosal Immunology, 2014, 7, 1283-1289. [Crossref], [Google Scholar], [Publisher], b) P.B. dos Santos Jr, Conceição de Maria Sales da Silva; Maria Elizabeth Gemaque Costa; Marcelo Costa Santos; Sergio Duvoisin Junior; Luiz Eduardo Pizarro Borges; Nélio Teixeira Machado. Kinetics of thermal degradation of PMMA-based dental resins scraps, Asian Journal of Green Chemistry, 2020, 4, 202-219 [Crossref], [Google Scholar], [Publisher], c) H.M. Bidhendi, Use chemical materials in automatic segmentation of teeth using x-ray, Advanced Journal of Chemistry-Section B: Natural Products and Medical Chemistry, 2023, 5, 1-13. [Crossref], [Pdf], [Publisher]
- d) S.J. AlKhalidy, K.S. Dosh, Isolation and purification of αs-cn from sheep milk and measuring the effectiveness of its enzymatic hydrolysis in inhibiting ACE1, 2023. [Crossref], [Google Scholar], [Publisher]
[14] L. Bracci-Laudiero, L. Aloe, P. Buanne, A. Finn, C. Stenfors, E. Vigneti, E. Theodorsson, T. Lundeberg, NGF modulates CGRP synthesis in human B-lymphocytes: a possible anti-inflammatory action of NGF, J Neuroimmunol, 2002, 123, 58-65 [Crossref], [Google Scholar], [Publisher]
[15] H. Wang, L. Xing, W. Li, L. Hou, J. Guo, X. Wang, Production and secretion of calcitonin gene-related peptide from human lymphocytes, J Neuroimmunol, 2002, 130, 155-162. [Crossref], [Google Scholar], [Publisher]
[16] L. Bracci-Laudiero, L. Aloe, M.C. Caroleo, P. Buanne, N. Costa, G. Starace, T. Lundeberg, Endogenous NGF regulates CGRP expression in human monocytes, and affects HLA-DR and CD86 expression and IL-10 production, Blood, 2005, 106, 3507-3514. [Crossref], [Google Scholar], [Publisher]
[17] W. Ma, R. Quirion, Increased calcitonin gene-related peptide in neuroma and invading macrophages is involved in the up-regulation of interleukin-6 and nerve injury associated thermal hyperalgesia in a rat model of mononeuropathy, J Neurochem, 2006, 98, 180–192. [Crossref], [Google Scholar], [Publisher]
[18] W. Ma, Y. Dumont, F. Vercauteren, R. Quirion, Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line, Immunology, 2010, 130, 399–409. [Crossref], [Google Scholar], [Publisher]
[19] S. Iyengar, M. H. Ossipov, K. W. Johnson, The Role of Calcitonin Gene–related Peptide in Peripheral and Central Pain Mechanisms Including Migraine, PAIN, 2017, 158, 543–559. [Crossref], [Google Scholar], [Publisher]
[20] K. Rajneesh, R. Bolash, Pathways of Pain Perception and Modulation, Fundamentals of Pain Medicine, 2018, 7-11. [Crossref], [Google Scholar], [Publisher]
[21] S. Hameed, Nav1.7 and Nav1.8: Role in the pathophysiology of pain, Molecular pain, 2019, 15, 1-11. [Crossref], [Google Scholar], [Publisher]
[22] F.A. Pinho-Ribeiro, W.A. Verri, I.M. Chiu, Nociceptor sensory neuron–immune interactions in pain and inflammation, Trends in Immunology, 2017, 38, 5–19. [Crossref], [Google Scholar], [Publisher]
[23] I. Rotstein, J.I. Ingle, Ingle's Endodontics 7th edition, 2019, 201-2012. [Publisher]
[24] A. Suwanchai, U. Theerapiboon, N. Chattipakorn, S. C. Chattipakorn, NaV 1.8, but not NaV 1.9, is upregulated in the inflamed dental pulp tissue of human primary teeth, Int Endod J, 2012, 45, 372-378. [Crossref], [Google Scholar], [Publisher]
[25] C.A. Warren, L. Mok, S. Gordon, A.F. Fouad, M.S. Gold, Quantification of neural protein in extirpated tooth pulp, J Endod, 2008, 34, 7-10. [Crossref], [Google Scholar], [Publisher]
[26] L. Jia, S. Lee, J.A. Tierney, J.K. Elmquist, M.D. Burton, L. Gautron, TLR4 signaling selectively and directly promotes CGRP release from vagal afferents in the mouse, ENEURO, 2020, 1-59. [Crossref], [Google Scholar], [Publisher]
[27] G. Sampoerno, A. Bhardwaj, P.Y. Divina, N.N. Fripertiwi, N.H. Adipradana, Neurogenic inflammation pathway on the up-regulation of voltage-gated sodium channel NaV1.7 in experimental flare-up post-dental pulp tissue extirpation, Journal of International Dental and Medical Research, 2022, 15, 124-130. [Google Scholar], [Publisher]
[28] A. Kaewpitak, C.S. Bauer, E.P. Seward, F.M. Boissonade, C.W.I. Douglas, Porphyromonas gingivalis lipopolysaccharide rapidly activates trigeminal sensory neurons and may contribute to pulpal pain, Int Endod J, 2020, 53, 846-858. [Crossref], [Google Scholar], [Publisher]
[29] B.E. Castillo-Silva, V. Martinez-Jimenez, G.A. Martinez-Castanon, C.E. Medina-Solis, E.C. Aguirre-Lopez, J.R. Castillo-Hernandes, N. Pation-Marin, Expression of calcitonin gene-related peptide and pulp sensitivity tests in irreversible pulpitis, Brazilian Oral Research, 2019, 33, 1-10. [Crossref], [Google Scholar], [Publisher]
[30] M. Yin, C. Li, X. D. Peng, G. Q. Zhao, Y. Wu, H.R. Zheng, Q. Wang, Q. Xu, N. Jiang, Expression and role of calcitonin gene-related peptide in mouse Aspergillus fumigatus keratitis, Int J Ophthalmol, 2019, 12, 697-704. [Crossref], [Google Scholar], [Publisher]
[31] a) J.-X. Duan, Y. Zhou, A.-Y. Zhou, X.-X. Guan, T. Liu, H.-H. Yang, P. Chen, Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro, Molecular Immunology, 2017, 91, 105–113. [Crossref], [Google Scholar], [Publisher], b) S.H. Abdullahi, A. Uzairu, G.A. Shallangwa, S. Uba, A. Umar, Pharmacokinetics studies of some diaryl pyrimidinamine derivatives as anti-cancer agent: in-silico drug design and molecular docking, Adv J Chem A, 2022, 5, 320-332. [Crossref], [Google Scholar], [Publisher]
[32] S. Hannoodee, D.N. Nasuruddin, Acute inflammatory response, StatPearls Publishing, 2020. [Google Scholar], [Publisher]
[33] T. Tajima, T. Murata, K. Aritake, Y. Urade, H. Hirai, M. Nakamura, M. Hori, Lipopolysaccharide Induces Macrophage Migration via Prostaglandin D2 and Prostaglandin E2, Journal of Pharmacology and Experimental Therapeutics, 2008, 326, 493-501. [Crossref], [Google Scholar], [Publisher]
[34] G. Natura, G.S. von Banchet, H.-G. Schaible, Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats, Pain, 2005, 116, 194–204. [Crossref], [Google Scholar], [Publisher]
[35] T. Renton, Y. Yiangou, C. Plumpton, S. Tate, C. Bountra, P. Anand, Sodium channel Nav1.8 immunoreactivity in painful human dental pulp, BMC Oral Health, 2005, 5, 5. [Crossref], [Google Scholar], [Publisher]
[36] E. V. Bird, C.R. Christmas, A.R. Loescher, K.G. Smith, P.P. Robinson, J.A. Black, S.G. Waxman, F.M. Boissonade, Correlation of Nav1.8 and Nav1.9 sodium channel expression with neuropathic pain in human subjects with lingual nerve neuromas, Mol Pain, 2013, 9, 52. [Crossref], [Google Scholar], [Publisher]
[37] F.A. Russell, R. King, S.J. Smillie, X. Kodji, S.D. Brain, Calcitonin gene-related peptide: physiology and pathophysiology, Physiol Rev, 2014, 94, 1099-1241. [Crossref], [Google Scholar], [Publisher]
[38] L. Xing, J. Guo, X. Wang, Induction and expression of beta-calcitonin gene-related peptide in rat T lymphocytes and its significance, J Immunol, 2000, 165, 4359-4366. [Crossref], [Google Scholar], [Publisher]
[39] Q. Hou, T. Barr, L. Gee, J. Vickers, J. Wymer, E. Borsani, L. Rodella, S. Getsios, T. Burdo, E. Eisenberg, U. Guha, R. Lavker, J. Kessler, S. Chittur, D. Fiorino, F. Rice, P. Albrecht, Keratinocyte expression of calcitonin gene-related peptide beta: implications for neuropathic and inflammatory pain mechanisms, Pain, 2011, 152, 2036–2051. [Crossref], [Google Scholar], [Publisher]
[40] R. Hu, Y. J. Li, X. H. Li, An Overview of Non-Neural Sources of Calcitonin Gene- Related Peptide, Current Medicinal Chemistry, 2016, 23, 763-773. [Google Scholar], [Publisher]

.png)
.png)
