Document Type : Original Research Article
Authors
1 Department of Environmental Science, Gadjah Mada University, Yogyakarta 55281, Indonesia
2 Department of Family and Community Medicine, Faculty of Medicine, Public Health and Nursing, Gadjah Mada University, Yogyakarta 55284, Indonesia
3 Faculty of Geography, Gadjah Mada University, Yogyakarta 55281, Indonesia
4 Department of Parasitology, Faculty of Medicine, Public Health and Nursing, Gadjah Mada University, Yogyakarta 55284, Indonesia
Abstract
Dengue fever remains a health issue in Indonesia, especially in Magetan Regency. Management of dengue disease is still aimed at controlling Aedes aegypti using malathion and alpha-cypermethrin. In Magetan Regency, there has been no report regarding the vulnerability status of Aedes mosquitoes to insecticides or mapping of the susceptibility status of Aedes to these insecticides. The aim of this study is to determine the distribution map of dengue cases and the susceptibility status of Aedes to malathion and Alfa-cypermethrin in four working areas of Community Health Centers in Magetan District, namely Candirejo, Taji, Plaosan, and Ngujung. The Arcgis application allows for the display of data in both table and map formats. This research uses an observation method with a cross-sectional study. Test sample collection was carried out in 400 houses in 4 work areas of community health centers with dengue fever problems in Magetan district, which were selected purposefully. The eggs and larvae obtained are kept in the laboratory. Based on the results, the population of Aedes in four health center areas showed resistance to malathion, with mortality rates of 25% in Ngujung, 52.5% in Plaosan, 48.33% in Candirejo, and 79.16% in Taji. Meanwhile, susceptibility to alpha-cypermethrin showed resistance in Candirejo and Plaosan, with mortality rates of 74.16% and 41.66%, respectively, and tolerance in Taji and Ngujung, with mortality rates of 87.5% and 93.33%, respectively. It can be concluded that resistance has occurred against organophosphate and pyrethroid insecticides.
Graphical Abstract
Keywords
Main Subjects
Introduction
Dengue, an infectious disease transmitted by the Aedes mosquito, is endemic in over 100 countries globally, particularly in Southeast Asia, the Americas, and the Western Pacific, posing a persistent public health challenge for the past seven decades [1]. In Indonesia, the reported number of dengue cases in 2021 reached 73,518, with an average incidence rate (IR) of 27 cases per 100,000 population and a case fatality rate (CFR) of 0.96% [2]. During the Covid-19 pandemic in 2020, 475 districts/cities in Indonesia were affected by Dengue Hemorrhagic Fever (DHF), as reported by the Ministry of Health of the Republic of Indonesia. Magetan District in East Java is one such DHF-endemic area. In 2021, Magetan Regency reported 218 DHF cases, with an incidence rate (IR) of 34.3 per 100,000 population and a mortality rate of 1.4% [3]. Vector control serves as a crucial strategy in interrupting the transmission chain of dengue, given the absence of a definitive cure or an optimal vaccine for the disease. Dengue Hemorrhagic Fever (DHF) vectors can be managed through physical, biological, and chemical control methods, with chemical control being a last resort due to its substantial impact. Nonetheless, it remains a primary tool for DHF vector control [4]. Among the chemical control measures, the application of insecticides through space spraying (thermal fogging/fogging or Ultra Low Volume/ULV) is a notable approach. A commonly utilized insecticide for fogging is malathion, belonging to the organophosphate group, and has served as a principal component in fogging formulations nationwide in Indonesia for numerous years [5].
In several regions of Indonesia, including Magetan District, pyrethroid insecticides, specifically Alpha Cypermethrin, have been incorporated into fogging practices since 2009. The recurrent and sustained use of insecticides within an ecosystem can induce vulnerability or susceptibility among vectors to the utilized insecticides [6,7]. The vulnerability emergence poses significant challenges, as resistant insects proliferate, leading to genetic alterations that diminish the resistance in subsequent generations, ultimately elevating the prevalence of resistant vectors in the population.
Ariati et.al, 2019 study in Indonesia revealed the susceptibility of Aedes aegypti mosquitoes to various insecticides, including Test results on 0.8% malathion revealed that 84% districts were resistant, 13% districts were tolerant, and 3% districts were vulnerable. While for 0.05% cypermethrin insecticide test; 98% were resistant, and 1% was tolerant. The test on 0.025% alpha cypermethrin found that 40% were resistant, 51% were tolerant and 9% were vulnerable.[8]. Similarly, research by Cahyati & Fitriani in 2020, conducted in Kupang Village, Panjang Village, and Tambakboyo Village in Ambarawa District, Semarang Regency, indicated that 27 out of 30 Aedes aegypti respondents exhibited susceptibility to the insecticide Cypermethrin [9]. The detection of vector susceptibility to insecticides provides valuable insights for designing targeted insecticide selection programs in vector control initiatives. Given the aforementioned context, it becomes imperative to ascertain the susceptibility status of Aedes aegypti to the insecticides employed in the dengue vector control program within Magetan District. The primary objective of this study is to delineate the susceptibility status and create a susceptibility map for vector species in areas with analogous epidemiological profiles, encompassing both past and prospective insecticide applications for vector control[10]. The outcomes of this research are anticipated to serve as crucial information, guiding policy decisions and evaluations in the ongoing efforts of vector control aimed at mitigating Dengue Hemorrhagic Fever (DHF) in Magetan District, thereby assisting the Magetan District Health Office in formulating effective strategies.
Experimental methods
The method employed in this research was observational method with a cross-sectional study, situated in four Health Center (Puskesmas) areas within Magetan Regency, East Java: Candirejo, Taji, Plaosan, and Ngujung Puskesmas areas. Entomological surveys involved purposively sampling Aedes aegypti larvae, and eggs, from 400 selected houses across the four health centers areas. One hundred houses were selected from each health center area, with activities including GPS-recorded coordinates of case houses, larva surveys, and the collection of larvae using a pipette. February to July was the duration of the study 2023 and received an ethical approval from the Ethics Committee of the Agency for Health Research (No. KE/FK/0172/EC/2023 dated 6 February 2023). Ovitraps were deployed for seven days to collect eggs. Larvae and pupae obtained from larval surveys, as well as from hatching eggs, are then reared in the laboratory until they become mosquitoes and their species can be identified based on WHO identification keys. Male and female Aedes aegypti mosquitoes are kept in a cube- shaped mosquito cage. Every day mosquitoes are fed a 10% sugar solution, and after 3-5 days the female mosquitoes are fed blood to mature their eggs. An ovitrap containing an ovistrip and water is provided in the cage, and the eggs attached to the ovistrip are harvested once a week and replaced with new ovistrips. Larvae from hatching eggs, which were then reared in the laboratory to obtain F1 progeny mosquito specimens for subsequent experiments in the laboratory. Adult mosquitoes are used for susceptibility testing aged 3-4 days, fed with sugar solution, were exposed to malathion (50 µg/bottle) and alpha-cypermethrin (10 µg/bottle) insecticides for 30 minutes, extending up to 2 hours. Knockdown observations were conducted every 15 minutes during the 2-hour exposure period, and mortality rates were calculated after 24 hours. Each test provides a control group without insecticide. If there is mortality above 20% in the control group then the data cannot be used. If there is mortality between 5-10% in the control group, and then the mortality data is corrected using the Abbot’s Formula. Mortality data was classified to determine the susceptibility status of the Aedes aegypti mosquito to the insecticides tested. Results are categorized as susceptible if the mosquito mortality rate is (98-100%), tolerant if the mosquito mortality rate is (80-97%), and resistant if the mosquito mortality rate is (< 80%), in line with the classification standards set by the Center for Control and Disease Prevention (CDC) [11].
Spatial analysis
A spatial analysis was conducted using ArcGIS software. The results were visualized within a geographic information system (GIS) environment in the form of maps.
Results and discussion
The findings indicated that Aedes aegypti populations in the four health centre areas showed resistance to Malathion, with a mortality rate of 25% in Ngujung, 52.5% in Plaosan, 48.33% in Candirejo, and 78.17% in Taji.While susceptibility to Alfa-sipermetrin showed resistance in Candirejo and Plaosan and Ngujung, with mortality rates of 74.17% and 65.00% and 68.33%respectively, and tolerance in Taji, with mortality rates of 82.5%. The percentage mortality of the test mosquitoes to malathion and alpha cypermetrin can be seen in Table 1.
Statistical analysis
The results of the Shapiro Wilk normality test on mosquito mortality data after exposure to the malathion and alpha-cypermethrin insecticides showed that the data were normally distributed (P>0.05), and based on the results of the Levene's test homogeneity showed that the data were homogeneous ( P>0.05), so statistical analysis was continued with TWO-way ANOVA. The results of the Two-way ANOVA statistical test are presented in Table 2.
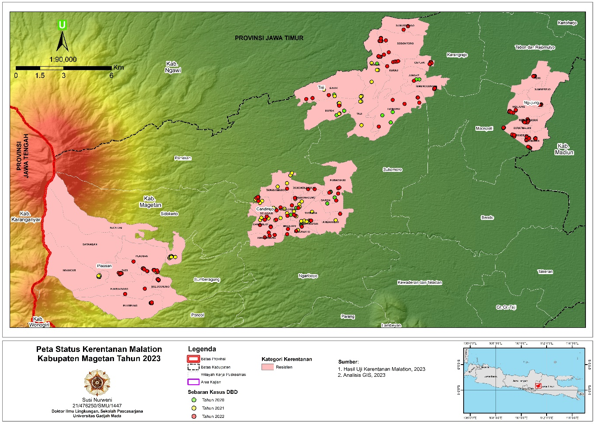
The results of the Two-way ANOVA test showed that there were differences in the types of insecticides, different locations, and the interaction between insecticides and locations (p<0.05). Statistical analysis was continued with the Duncan Multiple Range Test (DMRT).The results of statistical analysis with DMRT on the group of mosquitoes exposed to malathion showed that the highesta mortality rate was Aedes aegypti (F1) from the Taji Community Health Center Working Area, and the lowest mortality was Aedes aegypti from the Ngujung Community Health Center Working Area (Table 1). This indicates that the resistance status of Aedes aegypti (F1) from the Taji Community Health Center Working Area is the lowest and even close to tolerant because its mortality is almost 80%, while Aedes aegypti from the Ngujung Community Health Center Working Area is the most resistant to malathion insecticide. The map of case distribution and susceptibility status of Aedes aegypti mosquitoes to malathion by bioassay from four health center areas in Magetan is displayed in Figure 1.
Figure 1 shows that the working area of the Candirejo and Taji Community Health Centers is an endemic area for dengue fever because in the last 3 years (2020, 2021, and 2022) there have been cases of dengue fever, while the working area of the Plaosan Community Health Center is sporadic because in the last three years there have only been dengue cases in 2021 and 2022. Likewise, Ngujung is sporadic because there will only be dengue cases in 2022. Aedes aegypti from both dengue endemic areas (Candirejo and Taji) and dengue sporadic areas (Plaosan and Ngujung) is resistant to the malathion insecticide. The figure also shows that the Candirejo Health Centre's working area is resistant to malathion. Reported in 2020 there were 53 cases, 2021 there were 39 cases and 36 cases in 2022 while in the working area of Puskesmas Plaosan although it is resistant to malathion, new cases were reported in 2021 as many as 3 cases and in 2022, there were 7 cases.
The Taji Health Centre working area is also a malathion resistant area, reported in 2020, there were 10 cases, in 2021, there were 11 cases and there were 27 cases in 2023. Similarly, the Ngujung Health Centre working area is malation resistant, with 13 cases reported in 2022.
The results of statistical analysis using DMRT on the mortality rate of Aedes aegypti exposed to the alpha cypermethrin insecticide showed no significant difference, however, based on the results of the CDC Bottle Bioassay susceptibility test method, Aedes aegypti (F1) from the Taji Community Health Center Working Area was categorized as tolerant because its mortality was above 80% <98%. Mosquitoes from other Community Health Center Working Areas (Candirejo, Plaosan, and Ngujung) are categorized as resistant to the alpha-cypermethrin insecticide (Table 1). The map of case distribution and susceptibility status of Aedes aegypti mosquitoes to alpha-cypermethrin by bioassay from four health center areas in Magetan is depicted in Figure 2.
Figure 2 shows that the Candirejo Health Centre working area is a resistant area to Alpha cipermethrin in 2020 there were 53 cases, 2021 there were 39 cases and 36 cases in 2022 while in the Plaosan Health Center working area, it is also a resistant area to Alpha cipermethrin, new cases were reported in 2021 as many as 3 cases and in 2022, there were 7 cases.
The Taji Health Centre working area, which is a tolerant area to Alpha cypermethrin, reported 10 cases in 2020, 11 cases in 2021 and 27 cases in 2022 while in the Ngujung Health Centre working area, a tolerant area to Alpha cipermethrin, only 13 cases were reported in 2022.
The susceptibility assessment of Aedes aegypti to malathion revealed susceptibility across all surveyed locations. Notably, susceptibility to Alpha-cypermethrin was identified in two Health Center (Puskesmas) areas, while the remaining two areas in Magetan District demonstrated tolerance. Malathion was the primary insecticide used for dengue fogging in the surveyed sites for over two decades before transitioning to Alpha-cypermethrin. Although alpha-cypermethrin has been in use for more than ten years, certain villages within the surveyed sites, particularly in the Ngujung Health Centre area, have not undergone annual fogging procedures.
Organophosphate insecticides, specifically Malathion, indicate resistance within the study site's mosquito population. This resistance suggests the presence of Aedes aegypti mosquitoes that are resilient to organophosphate insecticides, with a potential heritability of this resistance across successive generations [12]. Rodriguez et al. substantiated the transgenerational inheritance of susceptibility status, a phenomenon previously demonstrated in research on deltamethrin susceptibility in Aedes aegypti populations [13]. In addition, findings from a study in Malaysia revealed that Aedes aegypti mosquitoes, subjected to exposure and selection with malathion up to the 45th generation, exhibited a significantly elevated level of susceptibility to the insecticide. The observed increase in susceptibility was 3.24 times higher than that of the 0th generation, highlighting the cumulative impact of successive generations on susceptibility traits [14].
Aedes aegypti mosquitoes in the four Health Center (Puskesmas) areas have demonstrated resistance to the malathion insecticide, a phenomenon believed to be closely linked to its extensive and continuous use due to the consistent occurrence of dengue cases each year. This observation aligns with the theory attributing vector mosquito resistance to prolonged exposure during density control operations employing malathion in Magetan District. The continual usage of insecticides is likely to expedite the development of resistance, as supported by Lima et al. (2011), emphasizing that prolonged exposure to specific insecticides prompts the emergence of resistant strains in Aedes aegypti mosquitoes. This adaptation is attributed to the mosquito's ability to develop an immune system against frequently utilized insecticides, accompanied by an increase in the production of detoxification enzymes such as esterase, glutathione S-transferase, and modifications in insecticide receptors [15]. Notably, Yudhana et al.’s (2017) research in Tegaldlimo, Purwoharjo, and Banyuwangi sub-districts, East Java, reported resistance among Aedes aegypti mosquitoes in the region to malathion at a concentration of 0.8% [16].
Susceptibility testing utilizing a diagnostic dose of 10 µg/bottle of Alpha-cypermethrin for 30 minutes revealed varying degrees of resistance and tolerance among mosquitoes in the four health center working areas. In the Magetan District Health Center's operational zone for dengue hemorrhagic fever (DHF) vector control, malathion insecticide was initially employed, but since 2009, it has been replaced by alpha-cypermethrin. Fogging is carried out in response to each DHF case. The extended use of alpha-cypermethrin for over a decade is associated with a potential decrease in susceptibility within Aedes aegypti mosquito populations. Notably, according to Georghiou (1986), Sucipto, Kuswandi dan Siswanto (2015), Sudiharto, Udiyono dan Kusariana (2020), and Widiastuti dan Ikawati (2016), continuous and prolonged use of insecticides may lead to the development of resistance within 2-20 years [17-20].
Resistance investigations have been conducted in various provinces on Sumatra Island. According to Sunaryo dan Widiastuti (2018), studies on Aedes aegypti mosquitoes in North Sumatra Province and Jambi Province indicated resistance to active ingredients such as Malathion 0.8%, Cypermethrin 0.05%, and Lambda cyhalothrin, while showing ongoing tolerance to alpha-cypermethrin [21]. The survey results depicting resistance and tolerance do not imply uniform conditions across all villages regarding Aedes aegypti resistance. The localized nature of susceptibility may be influenced by factors such as the absence of consistent mosquito control applications in other areas or limited movement of resistant Aedes aegypti mosquitoes, which can contribute to the transmission of resistance. A Singaporean study revealed that dengue-sensitive and newly sensitive areas exhibited resistant vector mosquitoes. This phenomenon is attributed to the high mobility of people and goods, facilitating the transportation of resistant mosquitoes. Consequently, these resistant mosquitoes may mate with susceptible Aedes aegypti, ultimately fostering the development of resistant Aedes aegypti populations [22].
In vector control, the insecticides' mode of action is categorized into five groups, influencing the nervous system, impeding energy production, impacting the endocrine system, inhibiting cuticle production, and disrupting water balance. In terms of the mode of entry into the insect body, insecticides may penetrate through the cuticle (contact poison), the digestive organs (stomach poison), or the respiratory openings (respiratory poison). Among the insecticides commonly utilized in vector control activities, organophosphates find extensive application in space spraying, indoor residual spraying (IRS), and larvicidal operations. Notable examples include Malathion, Fenitrothion, Temephos, Methyl-pyrimifos, and others [10].
Organophosphates operate by inhibiting the enzyme hydroxy acetylcholinesterase (AChE), leading to the disruption of nerve impulse transmission. This inhibition occurs through the binding of the pesticide to the acetylcholinesterase enzyme. Prolonged exposure to organophosphate pesticides poses a potential risk of chronic poisoning and carcinogenic effects [23]. Acetylcholinesterase, present in both the central and peripheral nervous systems, plays a crucial role in hydrolyzing the neurotransmitter acetylcholine. Acetylcholine, found at nerve-muscle junctions, facilitates the transmission of nerve stimuli. Impaired acetylcholinesterase function results in prolonged acetylcholine presence at receptors, intensifying and prolonging the excitatory effects of cholinergic nerves in both pre- and post- ganglionic regions. Cholinergic receptors, categorized into muscarinic and nicotinic classes, elicit specific reactions. Nicotinic receptors stimulate autonomic ganglia and skeletal muscle receptors, while muscarinic receptors activate organ end effector cells in bronchial smooth muscle, salivary glands, and the sinoatrial node. Both receptor types are present in the central nervous system [24]. The mode of action for all organophosphate and carbamate pesticides remains uniform, involving the inhibition of nerve impulse transmission by binding to cholinesterase. This mechanism prevents the hydrolysis of acetylcholine [25,26].
Alpha-cypermethrin, classified as a pyrethroid insecticide, acts as an axononic poison, specifically targeting nerve fibers. The mode of action involves binding to a nerve protein known as the voltage-gated sodium channel. In normal conditions, this protein opens to stimulate the nerve and closes to halt the nerve signal. Pyrethroids, including Alpha-cypermethrin, interfere with this gate, preventing its usual closure and leading to prolonged nerve stimulation. Consequently, poisoned insects exhibit tremors and incoordinated movements [27].
The varied modes of insecticide entry into an insect's body highlight the complexity of their mechanisms. Vector susceptibility testing serves the purpose of establishing the status and susceptibility map of vector species to insecticides utilized in their distribution areas. This information serves as a foundational basis for the regulation of insecticide use in vector control efforts.
Conclusion
Aedes aegypti mosquitoes in four of the surveyed health centers exhibited resistance to Malathion, with three health centers displaying resistance and one demonstrating tolerance to Alpha-cypermethrin. The persistence of insecticide use over 2-20 years in a given area, coupled with the utilization of inappropriate doses and targets, is identified as contributing factors to the observed resistance. This is used as a basis for regulating the use of insecticides in vector control.
Acknowledgments
The authors express gratitude to the Department of Parasitology, FK-KMK UGM, especially the Head, research colleagues, and technicians for their valuable assistance in conducting the study. Special thanks are extended to the Head of the Magetan Regency Health Office, the health centers in the survey locations, and their dedicated staff for their support and collaboration throughout the study's implementation.
Funding
Research funded by the Indonesian Ministry of Health.
Authors' Contributions
Nurweni contributed to the design and implemention of this research. H. Koesnanto, P. Widayani, S.R. Umniyati involved in planning and supervised the work. All authors discussed the results and contributed to the final manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest in this manuscript.
Orcid:
S. Nurweni*: https://orcid.org/0000-0002-1220-070
H. Kusnanto: https://orcid.org/0000-0001-7052-651
P. Widayani: https://orcid.org/0000-0003-0891-2074
S.R. Umniyati: https://orcid.org/0000-0002-8132-2866
-------------------------------------------------------------------------
How to cite this article: S. Nurweni*, H. Kusnanto, P. Widayani, S.R. Umniyati, Mapping and susceptibility of aedes aegypti to alpha-cypermethrin and malathion in magetan regency, east java. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(10), 1485-1495. Link: https://jmpcr.samipubco.com/article_195164.html
-------------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
