Document Type : Original Research Article
Authors
1 Department of Conservative Dentistry, Faculty of Dentistry Airlangga University, Surabaya, Indonesia
2 Conservative Dentistry Specialist Program, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia
3 Undergraduate Program, Faculty of Dentistry Airlangga University, Surabaya, Indonesia
Abstract
An inflammatory cytokine called tumor necrosis factor alpha (TNFα) is responsible for coordinating the body's reaction to injury and illness. TNFα expression can cause nociceptors to become hypersensitive to pain in addition to promoting inflammation. Based on a neuroimmunological approach, the immunohistochemistry image was examined by looking at the expression of TNFα and Hsp10 in macrophages and voltage-gated sodium channel 1.7 (Nav1.7) in nerve cells. Fifteen Sprague Dawley mice were used in the laboratory experiment; the animals were split into three groups, each with five mice: a control group, a group that removed normal pulp tissue, and a group that removed inflammatory pulp tissue. Tissue samples were taken from dental apical field of the mandibular incisor. The samples were all analyzed by immunohistochemistry techniques. When compared to the normal pulp tissue extraction group, the inflamed pulp tissue extraction group revealed a substantial drop in Nav1.7 expression, suggesting that Nav1.7 expression can be disrupted by increased Hsp10 expression. It implies that the discomfort risk during pulp extraction surgery will be reduced if the tooth pulp is infected or inflamed. The pulp tissue experiences an upregulation of TNFα expression upon extraction. Pain is caused by an increase in TNFα expression, which also raises Nav1.7 expression via the TNFR pathway. In the meantime, removing inflammatory pulp tissue significantly increases Hsp10 expression, which results in a drop in TNFα and Nav1.7 expression. When normal pulp tissue is removed, the pain response is more intense than when infected or inflammatory pulp tissue is removed.
Graphical Abstract
Keywords
Introduction
Adult permanent teeth with irreversible pulpitis require immediate root canal therapy or endodontic treatment because the inflamed, critical pulp cannot recover. A root canal, also known as an endodontic therapy, is a medical operation used to treat pulp inflammation and necrotic pulp tissue brought on by dental caries or trauma. For endodontically treated teeth to support the outcome of a root canal, clinical and radiographic examination is necessary. The whole pulp tissue in the root canal must be removed, followed by cleaning and bioinert material restoration [1-3]. A root canal treatment involves a three-dimensional hermetic obturation after a combination chemical and mechanical technique to eradicate pulpal and periradicular illness and promote periradicular tissue healing and restoration [4].
Endodontic flare-ups, which may be excruciating for both the dentist and the patient, occur when endodontic therapy exacerbates symptoms like pain and swelling during or after the procedure. A flare-up is characterized as an abrupt worsening of periradicular pathosis following the start or continuation of endodontic therapy, necessitating an unforeseen patient visit and dental intervention [5,6]. Studies have demonstrated that various variables, including mechanical, chemical, and microbiological ones, contribute to flare-ups [4]. Between 3% to 58% of all endodontic patients have postoperative discomfort, which is a relatively common occurrence [7]. The greatest frequency of flare-ups occurs 24 hours following root canal therapy, per studies by Aoun et al. and Vieyra et al. [8,9].
The painful illness known as pulpitis, which is mostly caused by Gram-negative bacteria, is an inflammation of the tooth pulp. The Gram-negative bacterial outer membrane contains lipopolysaccharides, which contribute to the inflammation of the tooth pulp [10]. Since lipopolysaccharide (LPS) is the main component of the cell walls of gram-negative bacteria, it is well-known to be a powerful activator of monocytes and macrophages. In addition, LPS can induce an acute inflammatory response by inducing the release of a large number of inflammatory cytokines in a variety of cell types [11]. Interleukin (IL) 6, IL 8, TNFα, and matrix metallopeptidase (MMP) 9 were all significantly upregulated by LPS [12]. In vitro studies employ LPS to cause inflammation in human dental pulp cells [10].
Blocking the TNFα/TNFR1 pathway or lowering immune cell TNFα production may be helpful for the illness advancement during the early stages of immunological damage, when TNFα secretion increases significantly [13]. Multipurpose cytokine tumor necrosis factor α (TNFα) has a strong pro-inflammatory impact [14]. TNFα controls immune system development, cell survival signaling pathways, proliferation, and metabolism [15]. The transgenic mouse model (TNFαglo) can be utilized to analyze pulpitis discomfort since TNFα expression on its own can generate inflammation that is comparable to osteitis and pulpitis [16]. TNFα is distinct in that it uses its two receptors, TNFR1 and TNFR2, to activate pleiotropic signaling [17]. TNFα expression can cause nociceptors to become hypersensitive to pain in addition to promote inflammation [16]. The enhanced regulation of NaV1.7 in DRG rat nerve cells is caused by TNFα, and NF-κβ is the signaling pathway implicated in this process [18].
Heat shock proteins are found in cells regularly and are among the most conserved proteins in evolution. However, they become overexpressed in response to stressors such abrupt temperature changes. The primary function of heat shock proteins (Hsps), which are conserved molecules, is to aid in the folding of other proteins. Because they are especially significant cytoprotectors in cells under stress, most Hsps are generally stress-inducible. Certain Hsps are released to the cell exterior, especially in reaction to stress, according to recent research. They are therefore typically thought of as danger signaling biomarkers. Moreover, it has been discovered that Hsp10 is increased and has a separate role in immunological regulation. Furthermore, Hsp10 by itself plays a major role in shielding prokaryotic or eukaryotic cells from environmental stressors brought on by inflammation, infection, and other factors [19,20].
Research has indicated a connection between a tooth's pulpal condition and a flare-up that occurs after endodontic therapy [21]. Compared to teeth with necrotic pulp, teeth with living pulps exhibit a decreased frequency of flare-ups [22]. When viable pulp is treated with a root canal, the incidence and severity of post-endodontic pain are much higher than when pulp or tooth necrosis is treated with a root canal [23]. There is ongoing debate on the correlation between the severity of pain, the frequency of flare-ups following endodontic treatment, and the pulp's state (necrosis or vitality) [24]. The diagnosis is crucial in predicting when flare-ups may occur [25]. Significant alterations in the levels of NaV1.3, NaV1.5, and NaV1.7 are brought on by nerve damage. Following nerve damage, NaV1.7 is highly expressed in the spine and is connected with pain [26]. This work uses a neuroimmunological method based on TNFα, Hsp10 expression in macrophage cells, and NaV1.7 expression in nerve cells to examine the incidence of immunohistochemistry flare-ups in normal and inflammatory dental pulp following pulp tissue extraction.
Materials and methods
This study used an experimental laboratory design with a control group that was only used for the post-test. ANOVA was used to statistically examine the data. In this investigation, fifteen male Sprague Dawley mice were watched and given an appropriate setting for seven consecutive days to allow for adaption. The mice met the inclusion requirements by being in good condition, weighing 450 g, being 20 weeks old, and having fully erupted incisors. They also received normal maintenance and basic food (NIH #31 rodent diet). They were split up into three groups, each with five mice: a control group, a group that had normal pulp tissue extraction, and a group that had inflammatory pulp tissue extraction. The members of the treatment group were terminated concurrently with the control group, which did not receive any therapy. Before pulp extrusion, the mandibular incisors of Sprague Dawley mice were given intraperitoneal injections of ketamine (80 mg/kg) and xylaxine (10 mg/kg) diluted in sterile Phosphate Buffer Saline (PBS) for the normal pulp tissue extirpation group. To create a flat surface using a fissure bur (Dia-Burs TC-21 ISO 160/014 FG LOT D14G007800 MANI Inc 8-3 Kiyohara Industrial Park Utsunomia, Tochigi, Japan) and open the pulp chamber using a round bur using a high-speed handpiece pana-max NSK OM-T0307E Japan, the lower incisor of the pulp tissue extraction group was severed down to the level of the interdental papilla (3 mm). Using a barbed broach (VDW Gmbh-Bayerwaldstr. 15-81737 Munich, Germany), the tooth was extracted to a depth of approximately 11 mm. After inserting the barbed broach into the root canal and rotating it 360 degrees, the pulp tissue was removed. Since the greatest frequency of flare-up was thought to occur 0-24 h following root canal therapy before dropping, the tooth was thereafter sealed with glass ionomer cement and ended after 24 h [27].
An access opening was created on the lower incisor for the group that had the inflamed pulp tissue extraction before an intrapulpal injection of lipopolysaccharide (LPS) stock isolated from Porphyromonas gingivalis (Ultrapure lipopolysaccharide from Porphyromonas gingivalis-TLR4 ligand, Catalog # tlrl-ppglps. Version #14F18-MM. InvivoGen. 3950 Sorrento Valley Blvd. Suite 100 San Diego, CA 92121-USA) was given using a micropipette with 10 µl. Afterward, glass ionomer cement was used to seal the teeth. The pulp tissue was removed after 48 hours, which is the amount of time needed for LPS induction to cause acute inflammation. Following a 24 h period, the teeth were extracted and cemented using glass ionomer cement [27].
Each jaw piece was fixed in 10% neutral formalin buffer for 24 hours after the pulp tissue removal in the treatment group was finished. It was subsequently decalcified at 4% Diamine Tetraacetic Acid (EDTA) for 30 days before a paraffin block preparation was created. Mandibular incisor analytical units were removed and subjected to immunohistochemistry analysis. Placed on polysine slides, the 4 μm thick paraffin block tissue slice was heated for an entire night at 56-58 °C. By immersing the incision in 3% hydrogen peroxide for 30 minutes at room temperature, endogenous peroxide activity was eradicated. After deparaffinizing a 4 μm apical tissue incision in xylene and rehydrating it with a graded alcohol and water solution (xylene for 4 x 5 minutes, absolute alcohol for 5 minutes, 95% alcohol for 5 minutes, 70% alcohol for 5 minutes, and finally washing with running water for 5 minutes), anti-rat monoclonal antibodies against NaV1.7, Hsp10, and TNFα were used for immunohistochemical staining. A light microscope with a 1000x magnification was utilized for reading preparations [28].
Results
Tables 1, 2, and 3 provide the mean and standard deviation for each phrase. The Levene test revealed homogenous findings (p > 0.05) and the Kolmogorov Smirnov test revealed normal results (p > 0.05) for the examination data in all treatment and control groups. The homogeneity of homogeneous variants and the normal data distribution are present in every treatment group.
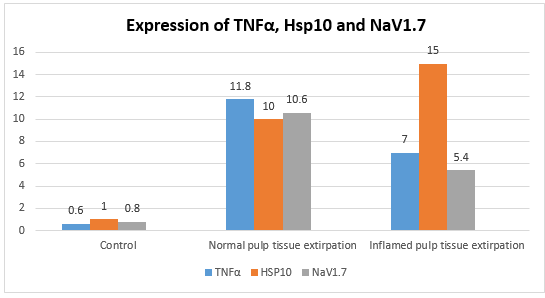
Table 1 shows that the Normal Pulp Tissue Extirpation group had the greatest mean expression of TNFα.
Figure 2 presents the findings. Table 2 indicates that the Inflamed Pulp Tissue Extirpation group had the greatest mean expression of HSP10. Figure 3 presents the findings. Table 3 shows that the Normal Pulp Tissue Extirpation group had the greatest mean expression of NaV1.7. Figure 4 displys the findings.
ANOVA statistical tests were used to ascertain the variations in TNFα expression, Hsp10, and NaV1.7 across treatment groups. The ANOVA test yielded a p value of 0.001 (p < 0.05). This demonstrates that there are notable variations in TNFα, Hsp10, and NaV1.7 expression throughout the therapy groups (Figure 1). The Tukey HSD statistical tests were run to determine whether treatment groups vary from one another. Table 4 presents the findings of the Tukey HSD statistical test. Table 4 shows that there is a p-value of less than 0.05 for TNFα expression across all treatment groups. This indicates that TNFα expression varies significantly across all treatments. The Hsp10 expression has the p-value less than 0.05 in every treatment group. This demonstrates that there are notable variations in Hsp10 expression across all treatment groups. P < 0.05 is seen for NaV1.7 expression across all treatment groups. This demonstrates that NaV1.7 expression varies significantly throughout all treatment groups.
Discussion
In this work, pulp tissue extraction in normal pulp results in an increase in TNFα expression. Damage associated molecular pattern (DAMP) is a result of cell damage in pulp tissue produced by extirpation, which increases the production of TNFα [29]. The expressions of Hsp70, NaV1.7, and CGRP were all elevated in the pulp tissue following its extraction. NaV1.7 may rise as a result of TNF being enriched by raising CGRP in nerve cells and Hsp70 in macrophage cells [30]. Release of ATP, HMGB1, DNA, HSP, and S100 are indicators of the DAMP. The surface of macrophage cells has a receptor called TLR-4 that detects the release of proteins from the cell. Moreover, it activates Myd 88, which in turn initiates an intracellular transduction signal, IRA activation, and the TRAF-6recruitment, which phosphorylates inhibitors of IKK, activating I-κB kinase and inhibiting I-κB, which in turn inactivates and activates NF-κB. TNFα is stimulated by NF-κB expression, leading to an increase in TNFα-expressing cells. Extracellular Hsp70 stimulates the inflammatory pathways by interacting with membrane receptors such as TLR2/4 or CD14. Lipopolysaccharides (LPS), a highly immunogenic bacterial endotoxin, may have been the cause of HSP70's pro-inflammatory characteristics [29].
Harmful pulp extraction-related trauma can raise HSP70. TNFα expression can be elevated by increased HSP70 from macrophages, which happens when pulp extirpation reacts to physical trauma [30]. TNFα increases trigger TNFR activation, which in turn triggers TRAF2 activation. MEK is activated by TRAF2 activation, which in turn activates MAPK. When MAPK is activated, NaV1.7 is activated as well, leading to an increase in NaV1.7 expression. Through methods that rely on the NF-κB and p38 MAPK signaling pathways in CNS neurons, TNFα increases Na+ currents by speeding up channel activation and boosting the expression of VGSC [31].
TNFα expression rises in normal pulp following pulp tissue extraction, which accounts for the increased expression of NaV1.7. TNF Receptor-Associated Receptor 2 (TRAF-2) can be activated by TNFα via TNF-R. Mitogen-activated Protein Kinase (MAPK) is triggered after TRAF-2 activation, which also activates MAPK Kinase (MEK). Sodium or sodium channels NaV1.7 are expressed when MAPK is activated. NaV1.7 expression rises in response to elevated TNFα expression. In rat neuropathic pain DRG neurons, exposure to TNFα increases sodium current [32]. NaV1.7 mRNA was upregulated by TNFα in both DRG neurons and adrenal chromaffin cells [33]. TNF increased NaV1.7 and raised the membrane p-p65, an active form of NF-kB, in cultured DRG neurons [34]. TNFα cytokine release, microglial cells regulate Na channels in bipolar neurons more intensely, and they form pyramids in a somewhat different way [35]. The sensitization process linked to neuropathic pain and inflammation is explained by the effect of TNFR activation on VGSC/NaV, which promotes excitation in primary afferent neurons [36].
Normal pulp exhibits increased expression of HSP10 following pulp tissue extraction because of its role in shielding cells from inflammatory and infectious stressors. Therefore, one of the intracellular stress proteins known as the resolution-associated molecular patterns (RAMPs) is binding immunoglobulin protein (BiP). When the RAMPs are decompartmentalized from inside the cell, they feed pro-resolution and anti-inflammatory signals into immune networks. In addition to be founding members of the RAMP family, HSP10, HSP27, and aB-crystallin share many of the extracellular immunomodulatory features of BiP [37].
After pulp tissue was removed from inflammatory pulp, Hsp10 expression was noticeably elevated. HSP10's extracellular appearance mediates its immunosuppressive properties. It increases the synthesis of the anti-inflammatory cytokine IL-10 while opposing the inflammatory cytokine production generated by LPS [38]. Hsp10 limits effector T cell infiltration into the spinal cord parenchyma in addition to downregulate the expression of adhesion molecules ICAM-1 and VCAM-1 as well as integrins LFA-1, VLA-4, and Mac-1 in the central nervous system (CNS) [39]. Hsp10 may target TLR signaling, preventing nuclear factor-κB activation produced by lipopolysaccharide (LPS) and the release of inflammatory mediators from human peripheral blood mononuclear cells and murine macrophages, including TNF-α, IL-6, and RANTES [38,19]. The expression of soluble TNFR, which binds to TNFα, is induced by an increase in HSP10. By competing with the cellular receptor species for TNF binding and maybe also functioning as dominant-negative molecules, the soluble TNF receptor variations block TNF [39]. As a result of the natural TNF buffering system's ability to regulate the runaway cytokine response, the concentration of soluble receptors rises after exposure to TNF generated during infections or upon the injection of recombinant TNF [40]. These investigations are consistent with the decline in TNFα and NaV1.7 expression following pulp tissue extraction from inflammatory pulp.
Conclusion
One of the root canal therapy procedures, pulp tissue extraction, generates trauma that increases the TNFα production in pulp tissue. Pain is brought on by an increase in TNFα, which also increases NaV1.7 expression via the TNFR pathway. Following pulp tissue extraction in inflamed pulp, NaV1.7 expression decreases as a result of a noticeably elevated expression of HSP10, which also causes a decrease in TNFα and NaV1.7 expression. In contrast to pulp extirpation after inflammatory dental pulp, a normal pulp tissue extirpation showed a more marked pain response based on its molecular nature.
Acknowledgements
The authors would like to thank the faculty of dental medicine Airlangga University for facilitating this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Galih Sampoerno conducts research and is the owner of the frame work; Nirawati Pribadi: manuscript editing; Arvia Diva Firstiana conducting research and manuscript editing; and Rijaal Daffa Ahmada: Statistic analysis.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Orcid:
Galih Sampoerno: https:.orcid.org/0000-0003-1437-3185
Nirawati Pribadi: https:.orcid.org/0000-0002-0930-3492
Arvia Diva Firstiana: https: orcid.org/0000-0003-4225-7907
-----------------------------------------------------------------------
How to cite this article: Galih Sampoerno*, Nirawati Pribadi, Arvia Diva Firstiana, Rijaal Daffa Ahmada, Expression of tnfα, hsp10, and nav1.7 in normal and inflamed dental pulp after pulp tissue extirpation. Journal of Medicinal and Pharmaceutical Chemistry Research, 2024, 6(3), 255-265. Link: http://jmpcr.samipubco.com/article_184196.html
-----------------------------------------------------------------------
Copyright © 2024 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
[1] a) L. M. Lin, D. Ricucci, T. M. Saoud, A. Sigurdsson, B. Kahler, Vital Pulp Therapy of Mature Permanent Teeth With Irreversible Pulpitis From The Perspective of Pulp Biology, Australian Endodontic Journal, 2020, 46, 154–166. [Crossref], [Google Scholar], [Publisher], b) H.M. Bidhendi, Use chemical materials in automatic segmentation of teeth using X-ray, Advanced Journal of Chemistry-Section B: Natural Products and Medical Chemistry, 2023, 5, 1-13. [Crossref], [Google Scholar], [Publisher]
[2] D. Sadaf, Success of coronal pulpotomy in permanent teeth with irreversible pulpitis: an evidence-based review, Cureus, 2020, 12, 23–27. [Crossref], [Google Scholar], [Publisher]
[3] S. Tabassum, F.R. Khan, Failure of endodontic treatment: The usual suspects, European journal of dentistry, 2016, 1,144–147. [Crossref], [Google Scholar], [Publisher]
[4] S. Bassam, R. El-Ahmar, S. Salloum, S. Ayoub, Endodontic postoperative flare-up: An update, The Saudi dental journal, 2021, 33, 7, 86-394. [Crossref], [Google Scholar], [Publisher]
[5] K.A. Lakshmi, G. U. Rao, M. Jayaraman, A. Ramdhas, Endodontic Flare-ups: A review, RGUHS Journal of Medical Sciences, 2020, 10, 9-17. [Crossref], [Google Scholar], [Publisher]
[6] J. Hegde, V. Prakash, R. Gupta, A. Srirekha, Concise conservative dentistry and Endodontics-E book, Elsevier Health Sciences, 2019, 628. [Google Scholar], [Publisher]
[7] O. Özdemir, Postoperative Pain in Endodontics, On J Dent & Oral Health, 2020, 3, 1-4. [Crossref], [Google Scholar], [Publisher]
[8] C. Aoun, N. El Costa, A. Naaman, C. Zogheib, I. Khalil, Post-endodontic flare-ups after a single-visit treatment using the FUI scoring method and associated factors: a clinical prospective study, Journal of Contemporary Dental Practice, 2019, 20, 1033–1040. [Google Scholar], [Publisher]
[9] J.P. Vieyra, F.J.J. Enriquez, F.O. Acosta, J.A. Guardado, Reduction of postendodontic pain after one-visit root canal treatment using three irrigating regimens with different temperature, Nigerian journal of clinical practice, 2019, 22, 34–40. [Crossref], [Google Scholar], [Publisher]
[10] A. Brodzikowska, M. Ciechanowska, M Kopka, A. Stachura, P.K. Włodarski, Role of lipopolysaccharide, derived from various bacterial species, in pulpitis-A systematic review, Biomolecules, 2022, 12, 138. [Crossref], [Google Scholar], [Publisher]
[11] M.M. Tucureanu, D. Rebleanu, C.A. Constantinescu, M. Deleanu, G. Voicu, E. Butoi, M. Calin, I. Manduteanu, Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor, International Journal of Nanomedicine, 2017, 20, 63-76. [Crossref], [Google Scholar], [Publisher]
[12] S. Zhao, J. Jiang, Y. Jing, W. Liu, X. Yang, X. Hou, L. Gao, L. Wei, The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity, Cell death & disease, 2020, 11, 1-13. [Crossref], [Google Scholar], [Publisher]
[13] M.S. Saddala, H. Huang, Identification of novel inhibitors for TNFα, TNFR1 and TNFα-TNFR1 complex using pharmacophore-based approaches, Journal of translational medicine, 2019, 17, 215. [Crossref], [Google Scholar], [Publisher]
[14] E. Varvolomeev, D. Vucic, Intracellular regulation of TNF activity in health and disease, Cytokine, 2018, 101, 26-32. [Crossref], [Google Scholar], [Publisher]
[15] P. Gough, I.A. Myles, Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects, Frontiers in immunology, 2020, 11,585880. [Crossref], [Google Scholar], [Publisher]
[16] B.E. Hall, L. Zhang, S.J. Sun, E. Utreras, M. Prochazkova, A. Cho, A. Terse, P. Arany, J.C. Dolan, B.C. Schmidt, A.B. Kulkarni, Conditional TNF-α overexpression in the tooth and alveolar bone results in painful pulpitis and osteitis, Journal of dental research, 2016, 95, 188–195. [Crossref], [Google Scholar], [Publisher]
[17] Y. Huang, Y. Zang, L. Zhou, W. Gui, Y. Zgong, The role of TNF-alpha/NF-kappa B pathway on the up-regulation of voltage-gated sodium channel Nav1. 7 in DRG neurons of rats with diabetic neuropathy, Neurochemistry International, 2014, 75, 112-119. [Crossref], [Google Scholar], [Publisher]
[18] S. Corrao, C. Campanella, R. Anzalone, F. Farina, G. Zummo, E. Conway de Macario, A.J. Macario, F. Cappello, G. La Rocca, Human Hsp10 and Early Pregnancy Factor (EPF) and their relationship and involvement in cancer and immunity: current knowledge and perspectives, Life sciences, 2010, 86, 145-152. [Crossref], [Google Scholar], [Publisher]
[19] T. Zininga, L. Ramatsui, A. Shonhai, Heat shock proteins as immunomodulants, Molecules, 2018, 23, 2846. [Crossref], [Google Scholar], [Publisher]
[20] A.A. Azim, K.A. Azim, P.V. Abbott, Prevalence of inter-appointment endodontic flare-ups and host-related factors, Clinical oral investigations, 2017, 21, 889–894. [Crossref], [Google Scholar], [Publisher]
[21] M. Gotler, G.B. Bar, M. Ashkenazi, Postoperative pain after root canal treatment: a prospective cohort study, International journal of dentistry, 2012, 310467. [Crossref], [Google Scholar], [Publisher]
[22] S. Elge, M. Rasmute, Pain and flare-up after endodontic treatment procedures, Stomatologija, 2014, 16, 25-30. [Google Scholar], [Publisher]
[23] M. Nair, J. Rahul, A. Devadathan, J. Mathew, Incidence of endodontic flare ups and its related factors: A retrospective study, Journal of Internasional Society of Preventive and Community Dentistry, 2017, 7, 175-
- [Crossref], [Google Scholar], [Publisher]
[24] J. Shao, J. Cao, J. Wang, X. Ren, S. Su, M. Li, Z. Li, Q. Zhao, W. Zang, MicroRNA-30b regulates expression of the sodium channel Nav1. 7 in nerve injury-induced neuropathic pain in the rat, Molecular pain, 2016, 12, 1744806916671523. [Crossref], [Google Scholar], [Publisher]
[25] G. Sampoerno, J. Sunariani, Kuntaman, Expression of NaV-1.7, TNF-α and HSP-70 in experimental flare-up post-extirpated dental pulp tissue through a neuroimmunological approach, The Saudi Dental Journal, 2020, 32, 206-218. [Crossref], [Google Scholar], [Publisher]
[26] Kim So-Woon, Park Jin Roh Chan-Sik. Immunohistochemistry for pathologists: protocols, pitfalls, and tips, Journal of pathology and translational medicine, 2016, 50, 411-419. [Crossref], [Google Scholar], [Publisher]
[27] G. Sampoerno, A. Bhardwaj, P.Y. Divina, N.N. Fripertiwi, N.H. Adipradana, Neurogenic Inflammation pathway on the up-regulation of voltage-gated sodium channel NaV1. 7 in experimental flare-up post-dental pulp tissue Extirpation, Journal of International Dental and Medical Research, 2022, 15, 124-130. [Google Scholar], [Publisher]
[28] S. Tukaj, Heat shock protein 70 as a double agent acting inside and outside the cell: insights into autoimmunity, International Journal of Molecular Sciences, 2020, 21, 5298. [Crossref], [Google Scholar], [Publisher]
[29] W. Chen, J. Sheng, J. Guo, F. Gao, X. Zhao, J. Dai, Tumor necrosis factor-α enhances voltage-gated Na+ currents in primary culture of mouse cortical neurons, Journal of neuroinflammation, 2015, 12, 1-10. [Crossref], [Google Scholar], [Publisher]
[30] F.H.P. Macedo, R.D. Aires, E.G. Fonseca, R.C.M. Ferreira, D.P.D. Machado, L. Chen, F.X. Zhang, I.A. Souza, V.S. Lemos, T.R.L. Romero, A. Moutal, R. Khanna, G.W. Zamponi, J.S. Cruz, TNF-α mediated upregulation of Na V 1.7 currents in rat dorsal root ganglion neurons is independent of CRMP2 SUMOylation, Molecular brain, 2019, 12, 1-14. [Crossref], [Google Scholar], [Publisher]
[31] R. Tamura, T. Nemoto, T. Maruta, S. Onizuka, T. Yanagita, A. Wada, I. Tsuneyoshi, Up-regulation of NaV1. 7 sodium channels expression by tumor necrosis factor-α in cultured bovine adrenal chromaffin cells and rat dorsal root ganglion neurons, Anesthesia & Analgesia, 2014, 118, 318–324. [Crossref], [Google Scholar], [Publisher]
[32] M.X. Xie, X.L. Zhang, J. Xu, W.A. Zeng, D. Li, T. Xu, R.P. Pang, K. Ma, X.G. Liu, Nuclear factor-kappaB gates Nav1. 7 channels in DRG neurons via protein-protein interaction, Iscience, 2019, 19, 623–633. [Google Scholar], [Publisher]
[33] L. Klapal, B.A. Igelhorst, I.D. Dietzel-Meyer, Changes in neuronal excitability by activated microglia: differential Na+ current upregulation in pyramid-shaped and bipolar neurons by TNF-α and IL-18, Frontiers in Neurology, 2016, 7, 44. [Crossref], [Google Scholar], [Publisher]
[34] M. Leo, S. Argalski, M. Schäfers, T. Hagenacker, Modulation of voltage-gated sodium channels by activation of tumor necrosis factor receptor-1 and receptor-2 in small DRG neurons of rats, Mediators of Inflammation, 2015, 124942. [Crossref], [Google Scholar], [Publisher]
[35] A.M. Shields, G.S. Panayi, V.M. Corrigall, Resolution-associated molecular patterns (RAMP): RAMParts defending immunological homeostasis?. Clinical & Experimental Immunology, 2011, 165, 292-300. [Crossref], [Google Scholar], [Publisher]
[36] A. Mázló, Y. Tang, V. Jenei, J. Brauman, H. Yousef, A. Bácsi, G. Koncz, Resolution Potential of Necrotic Cell Death Pathways, International Journal of Molecular Sciences, 2022, 24, 1, 16. [Crossref], [Google Scholar], [Publisher]
[37] B. Zhang, M.D. Walsh, K.B. Nguyen, N.C. Hillyard, A.C. Cavanagh, P.A. McCombe, H. Morton, Early pregnancy factor treatment suppresses the inflammatory response and adhesion molecule expression in the spinal cord of SJL/J mice with experimental autoimmune encephalomyelitis and the delayed-type hypersensitivity reaction to trinitrochlorobenzene in normal BALB/c mice, Journal of the neurological sciences, 2003, 212, 37-46. [Crossref], [Google Scholar], [Publisher]
[38] B.J. Johnson, T.T. Le, C.A. Dobbin, T. Banovic, C.B. Howard, M. Flores Fde, D. Vanags, D.J. Naylor, G.R. Hill, A. Suhrbier, Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production, Journal of Biological Chemistry, 2005, 280, 4037-4084. [Crossref], [Google Scholar], [Publisher]
[39] H. Wajant, D. Siegmund, TNFR1 and TNFR2 in the control of the life and death balance of macrophages, Frontiers in cell and developmental biology, 2019, 7, 91. [Crossref], [Google Scholar], [Publisher]
[40] S.F. Josephs, T.E. Ichim, S.M. Prince, S. Kesari, F.M. Marincola, A.R. Escobedo, A. Jafri, Unleashing endogenous TNF-alpha as a cancer immunotherapeutic, Journal of translational medicine, 2018, 16, 242. [Crossref], [Google Scholar], [Publisher]

.png)
_1.png)
.png)
.png)
.png)
